Structural basis for enhanced infectivity and immune evasion of SARS-CoV-2 variants
- PMID: 34168070
- PMCID: PMC9245151
- DOI: 10.1126/science.abi9745
Structural basis for enhanced infectivity and immune evasion of SARS-CoV-2 variants
Abstract
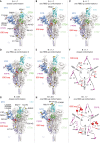
Several fast-spreading variants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have become the dominant circulating strains in the COVID-19 pandemic. We report here cryo-electron microscopy structures of the full-length spike (S) trimers of the B.1.1.7 and B.1.351 variants, as well as their biochemical and antigenic properties. Amino acid substitutions in the B.1.1.7 protein increase both the accessibility of its receptor binding domain and the binding affinity for receptor angiotensin-converting enzyme 2 (ACE2). The enhanced receptor engagement may account for the increased transmissibility. The B.1.351 variant has evolved to reshape antigenic surfaces of the major neutralizing sites on the S protein, making it resistant to some potent neutralizing antibodies. These findings provide structural details on how SARS-CoV-2 has evolved to enhance viral fitness and immune evasion.
Copyright © 2021, American Association for the Advancement of Science.
Figures



Update of
-
Structural basis for enhanced infectivity and immune evasion of SARS-CoV-2 variants.bioRxiv [Preprint]. 2021 Apr 14:2021.04.13.439709. doi: 10.1101/2021.04.13.439709. bioRxiv. 2021. Update in: Science. 2021 Aug 6;373(6555):642-648. doi: 10.1126/science.abi9745. PMID: 33880477 Free PMC article. Updated. Preprint.
References
-
- Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L., Chen H.-D., Chen J., Luo Y., Guo H., Jiang R.-D., Liu M.-Q., Chen Y., Shen X.-R., Wang X., Zheng X.-S., Zhao K., Chen Q.-J., Deng F., Liu L.-L., Yan B., Zhan F.-X., Wang Y.-Y., Xiao G.-F., Shi Z.-L., A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579, 270–273 (2020). 10.1038/s41586-020-2012-7 - DOI - PMC - PubMed
-
- H. Tegally, E. Wilkinson, M. Giovanetti, A. Iranzadeh, V. Fonseca, J. Giandhari, D. Doolabh, S. Pillay, E. J. San, N. Msomi, K. Mlisana, A. von Gottberg, S. Walaza, M. Allam, A. Ismail, T. Mohale, A. J. Glass, S. Engelbrecht, G. Van Zyl, W. Preiser, F. Petruccione, A. Sigal, D. Hardie, G. Marais, M. Hsiao, S. Korsman, M.-A. Davies, L. Tyers, I. Mudau, D. York, C. Maslo, D. Goedhals, S. Abrahams, O. Laguda-Akingba, A. Alisoltani-Dehkordi, A. Godzik, C. K. Wibmer, B. T. Sewell, J. Lourenço, L. C. J. Alcantara, S. L. Kosakovsky Pond, S. Weaver, D. Martin, R. J. Lessells, J. N. Bhiman, C. Williamson, T. de Oliveira, Emergence and rapid spread of a new severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) lineage with multiple spike mutations in South Africa. medRxiv 2020.12.21.20248640 [Preprint] (2020). 10.1101/2020.12.21.20248640. - DOI
-
- Voloch C. M., da Silva Francisco R. Jr., de Almeida L. G. P., Cardoso C. C., Brustolini O. J., Gerber A. L., Guimarães A. P. C., Mariani D., da Costa R. M., Ferreira O. C. Jr., Cavalcanti A. C., Frauches T. S., de Mello C. M. B., Leitão I. C., Galliez R. M., Faffe D. S., Castiñeiras T. M. P. P., Tanuri A., de Vasconcelos A. T. R.; Covid19-UFRJ Workgroup; LNCC Workgroup , Genomic characterization of a novel SARS-CoV-2 lineage from Rio de Janeiro, Brazil. J. Virol. 95, e00119–e00121 (2021). 10.1128/JVI.00119-21 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

