Dual oxidase 1 promotes antiviral innate immunity
- PMID: 34168077
- PMCID: PMC8256044
- DOI: 10.1073/pnas.2017130118
Dual oxidase 1 promotes antiviral innate immunity
Erratum in
-
Correction for Sarr et al., Dual oxidase 1 promotes antiviral innate immunity.Proc Natl Acad Sci U S A. 2021 Dec 7;118(49):e2119174118. doi: 10.1073/pnas.2119174118. Proc Natl Acad Sci U S A. 2021. PMID: 34845039 Free PMC article. No abstract available.
Abstract
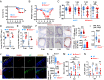
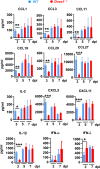
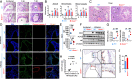
Dual oxidase 1 (DUOX1) is an NADPH oxidase that is highly expre-ssed in respiratory epithelial cells and produces H2O2 in the airway lumen. While a line of prior in vitro observations suggested that DUOX1 works in partnership with an airway peroxidase, lactoperoxidase (LPO), to produce antimicrobial hypothiocyanite (OSCN-) in the airways, the in vivo role of DUOX1 in mammalian organisms has remained unproven to date. Here, we show that Duox1 promotes antiviral innate immunity in vivo. Upon influenza airway challenge, Duox1-/- mice have enhanced mortality, morbidity, and impaired lung viral clearance. Duox1 increases the airway levels of several cytokines (IL-1β, IL-2, CCL1, CCL3, CCL11, CCL19, CCL20, CCL27, CXCL5, and CXCL11), contributes to innate immune cell recruitment, and affects epithelial apoptosis in the airways. In primary human tracheobronchial epithelial cells, OSCN- is generated by LPO using DUOX1-derived H2O2 and inactivates several influenza strains in vitro. We also show that OSCN- diminishes influenza replication and viral RNA synthesis in infected host cells that is inhibited by the H2O2 scavenger catalase. Binding of the influenza virus to host cells and viral entry are both reduced by OSCN- in an H2O2-dependent manner in vitro. OSCN- does not affect the neuraminidase activity or morphology of the influenza virus. Overall, this antiviral function of Duox1 identifies an in vivo role of this gene, defines the steps in the infection cycle targeted by OSCN-, and proposes that boosting this mechanism in vivo can have therapeutic potential in treating viral infections.
Keywords: DUOX1; Dual oxidase 1; hypothiocyanite; influenza; lactoperoxidase.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
-
- De Deken X., et al. ., Cloning of two human thyroid cDNAs encoding new members of the NADPH oxidase family. J. Biol. Chem. 275, 23227–23233 (2000). - PubMed
-
- Geiszt M., Witta J., Baffi J., Lekstrom K., Leto T. L., Dual oxidases represent novel hydrogen peroxide sources supporting mucosal surface host defense. FASEB J. 17, 1502–1504 (2003). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

