Interaction between the type 4 pili machinery and a diguanylate cyclase fine-tune c-di-GMP levels during early biofilm formation
- PMID: 34168081
- PMCID: PMC8256011
- DOI: 10.1073/pnas.2105566118
Interaction between the type 4 pili machinery and a diguanylate cyclase fine-tune c-di-GMP levels during early biofilm formation
Abstract
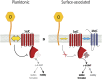
To initiate biofilm formation, it is critical for bacteria to sense a surface and respond precisely to activate downstream components of the biofilm program. Type 4 pili (T4P) and increasing levels of c-di-GMP have been shown to be important for surface sensing and biofilm formation, respectively; however, mechanisms important in modulating the levels of this dinucleotide molecule to define a precise output response are unknown. Here, using macroscopic bulk assays and single-cell tracking analyses of Pseudomonas aeruginosa, we uncover a role of the T4P alignment complex protein, PilO, in modulating the activity of the diguanylate cyclase (DGC) SadC. Two-hybrid and bimolecular fluorescence complementation assays, combined with genetic studies, are consistent with a model whereby PilO interacts with SadC and that the PilO-SadC interaction inhibits SadC's activity, resulting in decreased biofilm formation and increased motility. Using single-cell tracking, we monitor both the mean c-di-GMP and the variance of this dinucleotide in individual cells. Mutations that increase PilO-SadC interaction modestly, but significantly, decrease both the average and variance in c-di-GMP levels on a cell-by-cell basis, while mutants that disrupt PilO-SadC interaction increase the mean and variance of c-di-GMP levels. This work is consistent with a model wherein P. aeruginosa uses a component of the T4P scaffold to fine-tune the levels of this dinucleotide signal during surface commitment. Finally, given our previous findings linking SadC to the flagellar machinery, we propose that this DGC acts as a bridge to integrate T4P and flagellar-derived input signals during initial surface engagement.
Keywords: Pseudomonas aeruginosa; alignment complex; bacterial biofilms; c-di-GMP; surface sensing.
Conflict of interest statement
The authors declare no competing interest.
Figures


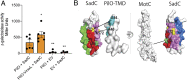

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

