Inverse and reciprocal regulation of p53/p21 and Bmi-1 modulates vasculogenic differentiation of dental pulp stem cells
- PMID: 34168122
- PMCID: PMC8225874
- DOI: 10.1038/s41419-021-03925-z
Inverse and reciprocal regulation of p53/p21 and Bmi-1 modulates vasculogenic differentiation of dental pulp stem cells
Abstract
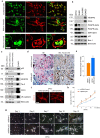
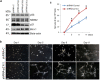
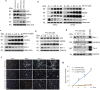
Dental pulp stem cells (DPSC) are capable of differentiating into vascular endothelial cells. Although the capacity of vascular endothelial growth factor (VEGF) to induce endothelial differentiation of stem cells is well established, mechanisms that maintain stemness and prevent vasculogenic differentiation remain unclear. Here, we tested the hypothesis that p53 signaling through p21 and Bmi-1 maintains stemness and inhibits vasculogenic differentiation. To address this hypothesis, we used primary human DPSC from permanent teeth and Stem cells from Human Exfoliated Deciduous (SHED) teeth as models of postnatal mesenchymal stem cells. DPSC seeded in biodegradable scaffolds and transplanted into immunodeficient mice generated mature human blood vessels invested with smooth muscle actin-positive mural cells. Knockdown of p53 was sufficient to induce vasculogenic differentiation of DPSC (without vasculogenic differentiation medium containing VEGF), as shown by increased expression of endothelial markers (VEGFR2, Tie-2, CD31, VE-cadherin), increased capillary sprouting in vitro; and increased DPSC-derived blood vessel density in vivo. Conversely, induction of p53 expression with small molecule inhibitors of the p53-MDM2 binding (MI-773, APG-115) was sufficient to inhibit VEGF-induced vasculogenic differentiation. Considering that p21 is a major downstream effector of p53, we knocked down p21 in DPSC and observed an increase in capillary sprouting that mimicked results observed when p53 was knocked down. Stabilization of ubiquitin activity was sufficient to induce p53 and p21 expression and reduce capillary sprouting. Interestingly, we observed an inverse and reciprocal correlation between p53/p21 and the expression of Bmi-1, a major regulator of stem cell self-renewal. Further, direct inhibition of Bmi-1 with PTC-209 resulted in blockade of capillary-like sprout formation. Collectively, these data demonstrate that p53/p21 functions through Bmi-1 to prevent the vasculogenic differentiation of DPSC.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

