High electrochemical and mechanical performance of zinc conducting-based gel polymer electrolytes
- PMID: 34168235
- PMCID: PMC8225769
- DOI: 10.1038/s41598-021-92671-5
High electrochemical and mechanical performance of zinc conducting-based gel polymer electrolytes
Abstract
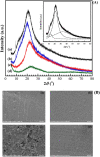
Zinc ionic conducting-based gel polymer electrolytes (GPEs) were fabricated from carboxymethyl cellulose (CMC) and three different zinc salts in a mass ratio ranging within 0-30 wt%. The effects of zinc salt and loading level on the structure, thermal, mechanical, mechanical stability, and morphological properties, as well as electrochemical properties of the GPEs films, were symmetrically investigated. The mechanical properties and mechanical stability of CMC were improved with the addition of zinc acetate, zinc sulphate, and zinc triflate, approaching the minimum requirement of a solid state membrane for battery. The maximum ionic conductivity of 2.10 mS cm-1 was achieved with the addition of 15 wt% zinc acetate (ZnA), GPEA15. The supported parameters, indicating the presence of the amorphous region that likely supported Zn2+ movement in the CMC chains, were clearly revealed with the increase in the number of mobile Zn2+ carriers in FT-IR spectra and the magnitude of ionic transference number, the decrease of the enthalpy of fusion in DSC thermogram, and the shifting to lower intensity of 2θ in XRD pattern. The developed CMC/ZnA complex-based GPEs are very promising for their high ionic conductivity as well as good mechanical properties and the ability for long-term utilization in a zinc ion battery.
Conflict of interest statement
The authors declare no competing interests.
Figures








References
-
- Li H, et al. Advanced rechargeable zinc-based batteries: Recent progress and future perspectives. Nano Energy. 2019;62:550–587. doi: 10.1016/j.nanoen.2019.05.059. - DOI
-
- Mainar AR, et al. An overview of progress in electrolytes for secondary zinc-air batteries and other storage systems based on zinc. J. Energy Storage. 2018;15:304–328. doi: 10.1016/j.est.2017.12.004. - DOI
-
- Rathika R, Padmaraj O, Suthanthiraraj SA. Electrical conductivity and dielectric relaxation behaviour of PEO/PVdF-based solid polymer blend electrolytes for zinc battery applications. Ionics. 2018;24:243–255. doi: 10.1007/s11581-017-2175-x. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

