The role of anterior insula-brainstem projections and alpha-1 noradrenergic receptors for compulsion-like and alcohol-only drinking
- PMID: 34168279
- PMCID: PMC8429444
- DOI: 10.1038/s41386-021-01071-w
The role of anterior insula-brainstem projections and alpha-1 noradrenergic receptors for compulsion-like and alcohol-only drinking
Abstract
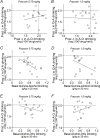
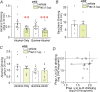
Compulsion-like alcohol drinking (CLAD), where consumption continues despite negative consequences, is a major obstacle to treating alcohol use disorder. The locus coeruleus area in the brainstem and norepinephrine receptor (NER) signaling in forebrain cortical regions have been implicated in adaptive responding under stress, which is conceptually similar to compulsion-like responding (adaptive responding despite the presence of stress or conflict). Thus, we examined whether anterior insula (aINS)-to-brainstem connections and alpha-1 NERs regulated compulsion-like intake and alcohol-only drinking (AOD). Halorhodopsin inhibition of aINS-brainstem significantly reduced CLAD, with no effect on alcohol-only or saccharin intake, suggesting a specific aINS-brainstem role in aversion-resistant drinking. In contrast, prazosin inhibition of alpha-1 NERs systemically reduced both CLAD and AOD. Similar to systemic inhibition, intra-aINS alpha-1-NER antagonism reduced both CLAD and AOD. Global aINS inhibition with GABAR agonists also strongly reduced both CLAD and AOD, without impacting saccharin intake or locomotion, while aINS inhibition of calcium-permeable AMPARs (with NASPM) reduced CLAD without impacting AOD. Finally, prazosin inhibition of CLAD and AOD was not correlated with each other, systemically or within aINS, suggesting the possibility that different aINS pathways regulate CLAD versus AOD, which will require further study to definitively address. Together, our results provide important new information showing that some aINS pathways (aINS-brainstem and NASPM-sensitive) specifically regulate compulsion-like alcohol consumption, while aINS more generally may contain parallel pathways promoting CLAD versus AOD. These findings also support the importance of the adaptive stress response system for multiple forms of alcohol drinking.
© 2021. The Author(s), under exclusive licence to American College of Neuropsychopharmacology.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

