A new high protein-to-energy enteral formula with a whey protein hydrolysate to achieve protein targets in critically ill patients: a prospective observational tolerability study
- PMID: 34168292
- PMCID: PMC8223230
- DOI: 10.1038/s41430-021-00956-9
A new high protein-to-energy enteral formula with a whey protein hydrolysate to achieve protein targets in critically ill patients: a prospective observational tolerability study
Abstract
Objectives: Current guidelines and expert recommendations stress the need to implement enteral feeds with a higher protein-to-energy ratio to meet protein requirements as recommended while avoiding gastrointestinal side effects and energy overfeeding in ICU patients.
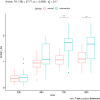
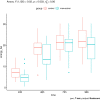
Materials and methods: Prospective tolerability study in 18 critically ill patients with a high protein formula (high protein-to-energy (HP:E) formula = Fresubin® Intensive; HPG) compared to a contemporary matched conventional therapy group (CTG). The primary outcome was GI intolerance defined as ≥300 ml daily gastric residual volume (GRV), vomiting, or diarrhea on days 1 and 2. Secondary outcomes were the percentage of patients reaching their protein target on day 4 and overall protein intake.
Results: Groups were comparable regarding demographic characteristics, disease severity, organ failures, mechanical ventilation, and NUTRIC score at baseline. Eighteen patients completed the 4-day feeding period. The number of events of GRV of ≥300 ml/day was equal in both groups (33.3%). The incidence of diarrhea and vomiting was low in the HPG (two patients concerned). EN did not need to be discontinued due to intolerance in any group. Seventy-two percent of patients reached protein targets ≥1.3 g/kgBW/d within 4 days after initiation of enteral feeding, which was superior to the CTG (33%). Post-hoc testing showed group differences of protein intake between HPG and CTG were significant at t = 72 h and t = 96 h. Energy targets were met in both groups.
Conclusion: The HP:E formula containing 33% whey protein hydrolysate is well tolerated in this tolerability study. Due to the HP:E ratio protein targets can be reached faster. Larger randomized trials are needed to confirm preliminary results.
Trial registration: ClinicalTrials.gov Identifier: NCT02678325. Registered 2 May 2016.
© 2021. The Author(s).
Conflict of interest statement
MS has received speaker honoraria from Fresenius Kabi, Switzerland. All contributing authors have not had any financial interest related to the study subject and report no conflicts of interest. This investigator-initiated study was funded by Fresenius-Kabi, Bad Homburg, Germany. The funder requested to start with a tolerability trial of 20 patients before further recruiting for the double-blind, randomized controlled clinical trial for comparison of the high protein enteral formula with a standard product on total calorie and protein intake. The funder was not involved in the study design otherwise, data collection, data analysis, and interpretation, writing of the manuscript, or the decision to submit the manuscript for publication. The manuscript was sent to Fresenius-Kabi before publication to determine whether it contained any confidential information or know-how. The authors take full responsibility for the content, completeness, correctness, and accuracy of the manuscript.
Figures

References
-
- Singer P, Blaser AR, Berger MM, Alhazzani W, Calder PC, Casaer MP, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38:48–79. - PubMed
-
- McClave SA, Taylor BE, Martindale RG, Warren MM, Johnson DR, Braunschweig C, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: society of critical care medicine (SCCM) and American society for parenteral and enteral nutrition (A.S.P.E.N.) JPEN J Parenter Enter Nutr. 2016;40:159–211. - PubMed
-
- Dhaliwal R, Cahill N, Lemieux M, Heyland DK. The Canadian critical care nutrition guidelines in 2013: an update on current recommendations and implementation strategies. Nutr Clin Pract. 2014;29:29–43. - PubMed
-
- Taylor S, Dumont N, Clemente R, Allan K, Downer C, Mitchell A. Critical care: meeting protein requirements without overfeeding energy. Clin Nutr ESPEN. 2016;11:e55–62. - PubMed
-
- Koekkoek K, van Zanten ARH. Nutrition in the ICU: new trends versus old-fashioned standard enteral feeding? Curr Opin Anaesthesiol. 2018;31:136–43. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

