Rapid generation of potent antibodies by autonomous hypermutation in yeast
- PMID: 34168368
- PMCID: PMC8463502
- DOI: 10.1038/s41589-021-00832-4
Rapid generation of potent antibodies by autonomous hypermutation in yeast
Abstract
The predominant approach for antibody generation remains animal immunization, which can yield exceptionally selective and potent antibody clones owing to the powerful evolutionary process of somatic hypermutation. However, animal immunization is inherently slow, not always accessible and poorly compatible with many antigens. Here, we describe 'autonomous hypermutation yeast surface display' (AHEAD), a synthetic recombinant antibody generation technology that imitates somatic hypermutation inside engineered yeast. By encoding antibody fragments on an error-prone orthogonal DNA replication system, surface-displayed antibody repertoires continuously mutate through simple cycles of yeast culturing and enrichment for antigen binding to produce high-affinity clones in as little as two weeks. We applied AHEAD to generate potent nanobodies against the SARS-CoV-2 S glycoprotein, a G-protein-coupled receptor and other targets, offering a template for streamlined antibody generation at large.
© 2021. The Author(s), under exclusive licence to Springer Nature America, Inc.
Figures


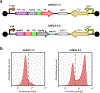





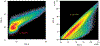
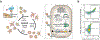



Update of
-
Rapid generation of potent antibodies by autonomous hypermutation in yeast.bioRxiv [Preprint]. 2020 Nov 11:2020.11.11.378778. doi: 10.1101/2020.11.11.378778. bioRxiv. 2020. Update in: Nat Chem Biol. 2021 Oct;17(10):1057-1064. doi: 10.1038/s41589-021-00832-4. PMID: 33200136 Free PMC article. Updated. Preprint.
Comment in
-
Full speed AHEAD in antibody discovery.Nat Chem Biol. 2021 Oct;17(10):1011-1012. doi: 10.1038/s41589-021-00838-y. Nat Chem Biol. 2021. PMID: 34211163 No abstract available.
References
Additional Online Methods References
Publication types
MeSH terms
Substances
Grants and funding
- T32 AI007245/AI/NIAID NIH HHS/United States
- COVID-19 Basic, Translational and Clinical Research Fund/UC | University of California, Irvine (UC Irvine)
- DP2 GM119163/GM/NIGMS NIH HHS/United States
- R01 AI146779/AI/NIAID NIH HHS/United States
- T32GM007753/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- Scholars Award/Vallee Foundation (Bert L. & N. Kuggie Vallee Foundation)
- T32 GM008313/GM/NIGMS NIH HHS/United States
- R35 GM136297/GM/NIGMS NIH HHS/United States
- 1R01CA260415/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- 1R35GM136297/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01AI146779/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- Inventors Fellowship/Gordon and Betty Moore Foundation (Gordon E. and Betty I. Moore Foundation)
- T32 GM007753/GM/NIGMS NIH HHS/United States
- R01 CA260415/CA/NCI NIH HHS/United States
- DP5 OD021345/OD/NIH HHS/United States
- DP5OD021345/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

