Fecal microbiota transplantation for irritable bowel syndrome: An intervention for the 21st century
- PMID: 34168399
- PMCID: PMC8192290
- DOI: 10.3748/wjg.v27.i22.2921
Fecal microbiota transplantation for irritable bowel syndrome: An intervention for the 21st century
Abstract
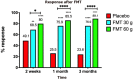
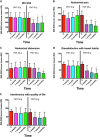
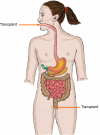
Irritable bowel syndrome (IBS) affects about 12% of the global population. Although IBS does not develop into a serious disease or increase mortality, it results in a considerable reduction in the quality of life. The etiology of IBS is not known, but the intestinal microbiota appears to play a pivotal role in its pathophysiology. There is no effective treatment for IBS, and so the applied treatments clinically focus on symptom relief. Fecal microbiota transplantation (FMT), an old Chinese treatment, has been applied to IBS patients in seven randomized controlled trials (RCTs). Positive effects on IBS symptoms in various degrees were obtained in four of these RCTs, while there was no effect in the remaining three. Across the seven RCTs there were marked differences in the selection processes for the donor and treated patients, the transplant dose, the route of administration, and the methods used to measure how the patients responded to FMT. The present frontier discusses these differences and proposes: (1) criteria for selecting an effective donor (superdonor); (2) selection criteria for patients that are suitable for FMT; (3) the optimal FMT dose; and (4) the route of transplant administration. FMT appears to be safe, with only mild, self-limiting side effects of abdominal pain, cramping, tenderness, diarrhea, and constipation. Although it is early to speculate about the mechanisms underlying the effects of FMT, the available data suggest that changes in the intestinal bacteria accompanied by changes in fermentation patterns and fermentation products (specifically short-chain fatty acids) play an important role in improving the IBS symptoms seen after FMT. FMT appears to be a promising treatment for IBS, but further studies are needed before it can be applied in everyday clinical practice.
Keywords: Butyric acid; Enteroendocrine cells; Etiology; Microbiota; Short-chain fatty acids; Superdonor; Therapy.
©The Author(s) 2021. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The authors declare that they have no conflict of interest.
Figures









References
-
- Konturek PC, Brzozowski T, Konturek SJ. Stress and the gut: pathophysiology, clinical consequences, diagnostic approach and treatment options. J Physiol Pharmacol. 2011;62:591–599. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

