Why Are Stroke Rehabilitation Trial Recruitment Rates in Single Digits?
- PMID: 34168611
- PMCID: PMC8217867
- DOI: 10.3389/fneur.2021.674237
Why Are Stroke Rehabilitation Trial Recruitment Rates in Single Digits?
Abstract
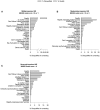
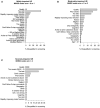
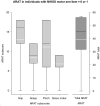
Background: Recruitment of patients in early subacute rehabilitation trials (<30 days post-stroke) presents unique challenges compared to conventional stroke trials recruiting individuals >6 months post-stroke. Preclinical studies suggest treatments be initiated sooner after stroke, thus requiring stroke rehabilitation trials be conducted within days post-stroke. How do specific inclusion and exclusion criteria affect trial recruitment rates for early stroke rehabilitation trials? Objectives: Provide estimates of trial recruitment based on screening and enrollment data from a phase II early stroke rehabilitation trial. Methods: CPASS, a phase II intervention trial screened ischemic stroke patients in acute care (18-months, N = 395) and inpatient rehabilitation (22-months, N = 673). Patients were stratified by upper extremity (UE) impairment into mild (NIHSS motor arm = 0, 1); moderate (NIHSS = 2, 3); severe (NIHSS = 4) and numbers of patients disqualified due to CPASS exclusion criteria determined. We also examined if a motor-specific evaluation (Action Research Arm Test, ARAT) increases the pool of eligible patients disqualified by the NIHSS motor arm item. Results: CPASS recruitment in acute care (5.3%) and inpatient rehabilitation (5%) was comparable to prior trials. In acute care, a short stay (7-17-days), prior stroke (13.5% in moderately; 13.2% in severely impaired) disqualified the majority. In inpatient rehabilitation, the majority (40.8%) were excluded for "too mild" impairment. The next majority were disqualified for reaching inpatient rehabilitation "too late" to participate in an early stroke trial (15% in moderately; 24% in severely impaired). Mean ARAT in the "too mild" showed significant impairment and potential to benefit from participation in select UE rehabilitation trials. Conclusions: Screening of ischemic stroke patients while they are still in acute care is crucial to successful recruitment for early stroke rehabilitation trials. A significant proportion of eligible patients are lost to "short length of stay" in acute care, and arrive to inpatient rehabilitation "too late" for an early rehabilitation trial. Additional screening of mildly impaired patients using a motor function specific scale will benefit the trial recruitment and generalizability. Trial Registration Number: http://www.clinicaltrials.gov Identifier: NCT02235974.
Keywords: clinical trial; prospective study; rehabilitation; stroke; trial design.
Copyright © 2021 Geed, Feit, Edwards and Dromerick.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Alcobendas-Maestro M, Esclarin-Ruz A, Casado-Lopez RM, Munoz-Gonzalez A, Perez-Mateos G, Gonzalez-Valdizan E, et al. . Lokomat robotic-assisted versus overground training within 3 to 6 months of incomplete spinal cord lesion: randomized controlled trial. Neurorehabil Neural Rep. (2012) 26:1058–63. 10.1177/1545968312448232 - DOI - PubMed
-
- Nadeau SE, Wu SS, Dobkin BH, Azen SP, Rose DK, Tilson JK, et al. . Effects of task-specific and impairment-based training compared with usual care on functional walking ability after inpatient stroke rehabilitation: LEAPS trial. Neurorehabil Neural Repair. (2013) 27:370–80. 10.1177/1545968313481284 - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

