Emergent synthetic methods for the modular advancement of sp3-rich fragments
- PMID: 34168751
- PMCID: PMC8179648
- DOI: 10.1039/d1sc00161b
Emergent synthetic methods for the modular advancement of sp3-rich fragments
Abstract
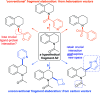
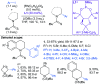
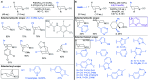
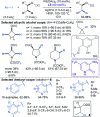
Fragment-based drug discovery is an important and increasingly reliable technology for the delivery of clinical candidates. Notably, however, sp3-rich fragments are a largely untapped resource in molecular discovery, in part due to the lack of general and suitably robust chemical methods available to aid their development into higher affinity lead and drug compounds. This Perspective describes the challenges associated with developing sp3-rich fragments, and succinctly highlights recent advances in C(sp3)-H functionalisations of high potential value towards advancing fragment hits by 'growing' functionalised rings and chains from unconventional, carbon-centred vectors.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures







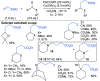
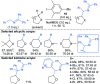


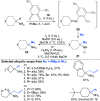

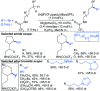


References
-
- Baker M. Fragment-Based Lead Discovery Grows Up. Nat. Rev. Drug Discovery. 2012;121:2012. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

