Photoactive electron donor-acceptor complex platform for Ni-mediated C(sp3)-C(sp2) bond formation
- PMID: 34168786
- PMCID: PMC8179655
- DOI: 10.1039/d1sc00943e
Photoactive electron donor-acceptor complex platform for Ni-mediated C(sp3)-C(sp2) bond formation
Abstract
A dual photochemical/nickel-mediated decarboxylative strategy for the assembly of C(sp3)-C(sp2) linkages is disclosed. Under light irradiation at 390 nm, commercially available and inexpensive Hantzsch ester (HE) functions as a potent organic photoreductant to deliver catalytically active Ni(0) species through single-electron transfer (SET) manifolds. As part of its dual role, the Hantzsch ester effects a decarboxylative-based radical generation through electron donor-acceptor (EDA) complex activation. This homogeneous, net-reductive platform bypasses the need for exogenous photocatalysts, stoichiometric metal reductants, and additives. Under this cross-electrophile paradigm, the coupling of diverse C(sp3)-centered radical architectures (including primary, secondary, stabilized benzylic, α-oxy, and α-amino systems) with (hetero)aryl bromides has been accomplished. The protocol proceeds under mild reaction conditions in the presence of sensitive functional groups and pharmaceutically relevant cores.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

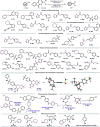


References
-
-
For selected examples see:
- Jana R. Pathak T. P. Sigman M. S. Chem. Rev. 2011;111:1417–1492. - PMC - PubMed
- Gildner P. G. Colacot T. J. Organometallics. 2015;34:5497–5508.
- Johansson Seechurn C. C. C. Kitching M. O. Colacot T. J. Snieckus V. Angew. Chem., Int. Ed. 2012;51:5062–5085. - PubMed
- Campeau L.-C. Hazari N. Organometallics. 2019;38:3–35. - PMC - PubMed
- Choi J. Fu G. C. Science. 2017;356:7230. - PMC - PubMed
- Gu J. Wang X. Xue W. Gong H. Org. Chem. Front. 2015;2:1411–1421.
- Hu X. Chem. Sci. 2011;2:1867–1886.
- Kaga A. Chiba S. ACS Catal. 2017;7:4697–4706.
- Ma X. Murray B. Biscoe M. R. Nat. Rev. Chem. 2020;4:584–599. - PMC - PubMed
- Weix D. J. Acc. Chem. Res. 2015;48:1767–1775. - PMC - PubMed
-
-
-
For selected examples see:
- Everson D. A. Shrestha R. Weix D. J. J. Am. Chem. Soc. 2010;132:920–921. - PubMed
- Everson D. A. Weix D. J. J. Org. Chem. 2014;79:4793–4798. - PMC - PubMed
- Gu J. Qiu C. Lu W. Qian Q. Lin K. Gong H. Synthesis. 2017;49:1867–1873.
- Yu X. Yang T. Wang S. Xu H. Gong H. Org. Lett. 2011;13:2138–2141. - PubMed
- Woods B. P. Orlandi M. Huang C.-Y. Sigman M. S. Doyle A. G. J. Am. Chem. Soc. 2017;139:5688–5691. - PubMed
- Jin Y. Yang H. Wang C. Org. Lett. 2019;21:7602–7608. - PubMed
- Kadunce N. T. Reisman S. E. J. Am. Chem. Soc. 2015;137:10480–10483. - PMC - PubMed
- Knappke C. E. I. Grupe S. Gärtner D. Corpet M. Gosmini C. Jacobi von Wangelin A. Chem.–Eur. J. 2014;20:6828–6842. - PubMed
- Moragas T. Correa A. Martin R. Chem.–Eur. J. 2014;20:8242–8258. - PubMed
- Tian Z.-X. Qiao J.-B. Xu G.-L. Pang X. Qi L. Ma W.-Y. Zhao Z.-Z. Duan J. Du Y.-F. Su P. Liu X.-Y. Shu X.-Z. J. Am. Chem. Soc. 2019;141:7637–7643. - PubMed
- Cherney A. H. Reisman S. E. J. Am. Chem. Soc. 2014;136:14365–14368. - PMC - PubMed
- Huihui K. M. M. Caputo J. A. Melchor Z. Olivares A. M. Spiewak A. M. Johnson K. A. DiBenedetto T. A. Kim S. Ackerman L. K. G. Weix D. J. J. Am. Chem. Soc. 2016;138:5016–5019. - PMC - PubMed
-
; for selected examples using electrochemical methods, see:
- Li H. Breen C. P. Seo H. Jamison T. F. Fang Y.-Q. Bio M. M. Org. Lett. 2018;20:1338–1341. - PMC - PubMed
- Koyanagi T. Herath A. Chong A. Ratnikov M. Valiere A. Chang J. Molteni V. Loren J. Org. Lett. 2019;21:816–820. - PubMed
-
; for cross-electrophile coupling in biological environments, see:
- Flood D. T. Asai S. Zhang X. Wang J. Yoon L. Adams Z. C. Dillingham B. C. Sanchez B. B. Vantourout J. C. Flanagan M. E. Piotrowski D. W. Richardson P. Green S. A. Shenvi R. A. Chen J. S. Baran P. S. Dawson P. E. J. Am. Chem. Soc. 2019;141:9998–10006. - PMC - PubMed
-
-
-
For selected examples on decarboxylative arylation using carbon preformed nucleophiles, see:
- Chen T.-G. Zhang H. Mykhailiuk P. K. Merchant R. R. Smith C. A. Qin T. Baran P. S. Angew. Chem., Int. Ed. 2019;58:2454–2458. - PMC - PubMed
- Cornella J. Edwards J. T. Qin T. Kawamura S. Wang J. Pan C.-M. Gianatassio R. Schmidt M. Eastgate M. D. Baran P. S. J. Am. Chem. Soc. 2016;138:2174–2177. - PMC - PubMed
- Liu X.-G. Zhou C.-J. Lin E. Han X.-L. Zhang S.-S. Li Q. Wang H. Angew. Chem., Int. Ed. 2018;57:13096–13100. - PubMed
- Toriyama F. Cornella J. Wimmer L. Chen T.-G. Dixon D. D. Creech G. Baran P. S. J. Am. Chem. Soc. 2016;138:11132–11135. - PMC - PubMed
-
-
- Kuroboshi M. Waki Y. Tanaka H. Synlett. 2002;2002:0637–0639.
- Kuroboshi M. Waki Y. Tanaka H. J. Org. Chem. 2003;68:3938–3942. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

