DNA nanostructure-based nucleic acid probes: construction and biological applications
- PMID: 34168817
- PMCID: PMC8188511
- DOI: 10.1039/d1sc00587a
DNA nanostructure-based nucleic acid probes: construction and biological applications
Abstract
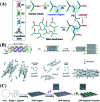
In recent years, DNA has been widely noted as a kind of material that can be used to construct building blocks for biosensing, in vivo imaging, drug development, and disease therapy because of its advantages of good biocompatibility and programmable properties. However, traditional DNA-based sensing processes are mostly achieved by random diffusion of free DNA probes, which were restricted by limited dynamics and relatively low efficiency. Moreover, in the application of biosystems, single-stranded DNA probes face challenges such as being difficult to internalize into cells and being easily decomposed in the cellular microenvironment. To overcome the above limitations, DNA nanostructure-based probes have attracted intense attention. This kind of probe showed a series of advantages compared to the conventional ones, including increased biostability, enhanced cell internalization efficiency, accelerated reaction rate, and amplified signal output, and thus improved in vitro and in vivo applications. Therefore, reviewing and summarizing the important roles of DNA nanostructures in improving biosensor design is very necessary for the development of DNA nanotechnology and its applications in biology and pharmacology. In this perspective, DNA nanostructure-based probes are reviewed and summarized from several aspects: probe classification according to the dimensions of DNA nanostructures (one, two, and three-dimensional nanostructures), the common connection modes between nucleic acid probes and DNA nanostructures, and the most important advantages of DNA self-assembled nanostructures in the applications of biosensing, imaging analysis, cell assembly, cell capture, and theranostics. Finally, the challenges and prospects for the future development of DNA nanostructure-based nucleic acid probes are also discussed.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures







References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

