Nanofibrils in nature and materials engineering
- PMID: 34168896
- PMCID: PMC8221570
- DOI: 10.1038/natrevmats.2018.16
Nanofibrils in nature and materials engineering
Abstract
Nanofibrillar materials, such as cellulose, chitin and silk, are highly ordered architectures, formed through the self-assembly of repetitive building blocks into higher-order structures, which are stabilized by non-covalent interactions. This hierarchical building principle endows many biological materials with remarkable mechanical strength, anisotropy, flexibility and optical properties, such as structural colour. These features make nanofibrillar biopolymers interesting candidates for the development of strong, sustainable and biocompatible materials for environmental, energy, optical and biomedical applications. However, recreating their architecture is challenging from an engineering perspective. Rational design approaches, applying a combination of theoretical and experimental protocols, have enabled the design of biopolymer-based materials through mimicking nature's multiscale assembly approach. In this Review, we summarize hierarchical design strategies of cellulose, silk and chitin, focusing on nanoconfinement, fibrillar orientation and alignment in 2D and 3D structures. These multiscale architectures are discussed in the context of mechanical and optical properties, and different fabrication strategies for the manufacturing of biopolymer nanofibril-based materials are investigated. We highlight the contribution of rational material design strategies to the development of mechanically anisotropic and responsive materials and examine the future of the material-by-design paradigm.
Conflict of interest statement
Competing interests The authors declare no competing interests.
Figures




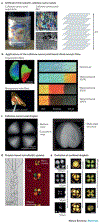
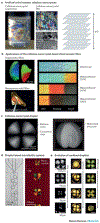
References
-
- Neville AC Biology of Fibrous Composites: Development Beyond the Cell Membrane (Cambridge Univ. Press, New York, 1993).
-
- Meyers MA & Chen PY in Biological Materials Science: Biological Materials, Bioinspired Materials, and Biomaterials 53–97 (Cambridge Univ. Press, Cambridge, 2014).
-
- Mitov M Cholesteric liquid crystals in living matter. Soft Matter 13, 4176–4209 (2017). - PubMed
-
- Moon RJ, Martini A, Nairn J, Simonsen J & Youngblood J Cellulose nanomaterials review: structure, properties and nanocomposites. Chem. Soc. Rev 40, 3941–3994 (2011). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
