Wiskott-Aldrich Syndrome Protein: Roles in Signal Transduction in T Cells
- PMID: 34169073
- PMCID: PMC8217661
- DOI: 10.3389/fcell.2021.674572
Wiskott-Aldrich Syndrome Protein: Roles in Signal Transduction in T Cells
Abstract
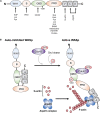
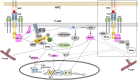
Signal transduction regulates the proper function of T cells in an immune response. Upon binding to its specific ligand associated with major histocompatibility complex (MHC) molecules on an antigen presenting cell, the T cell receptor (TCR) initiates intracellular signaling that leads to extensive actin polymerization. Wiskott-Aldrich syndrome protein (WASp) is one of the actin nucleation factors that is recruited to TCR microclusters, where it is activated and regulates actin network formation. Here we highlight the research that has focused on WASp-deficient T cells from both human and mice in TCR-mediated signal transduction. We discuss the role of WASp in proximal TCR signaling as well as in the Ras/Rac-MAPK (mitogen-activated protein kinase), PKC (protein kinase C) and Ca2+-mediated signaling pathways.
Keywords: MAPK; PKC; T cell activation; T cell signaling; WASp; calcium.
Copyright © 2021 Ngoenkam, Paensuwan, Wipa, Schamel and Pongcharoen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Amasaki Y., Masuda E. S., Imamura R., Arai K., Arai N. (1998). Distinct NFAT family proteins are involved in the nuclear NFAT-DNA binding complexes from human thymocyte subsets. J. Immunol. 160 2324–2333. - PubMed
-
- Badour K., Zhang J., Shi F., Leng Y., Collins M., Siminovitch K. A. (2004). Fyn and PTP-PEST-mediated regulation of Wiskott-Aldrich syndrome protein (WASp) tyrosine phosphorylation is required for coupling T cell antigen receptor engagement to WASp effector function and T cell activation. J. Exp. Med. 199 99–112. 10.1084/jem.20030976 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

