Paired Spectroscopic and Crystallographic Studies of Proteases
- PMID: 34169145
- PMCID: PMC8221577
- DOI: 10.1002/slct.201902049
Paired Spectroscopic and Crystallographic Studies of Proteases
Abstract
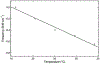
The active sites of subtilisin and trypsin have been studied by paired IR spectroscopic and X-ray crystallographic studies. The active site serines of the proteases were reacted with 4-cyanobenzenesulfonyl fluoride (CBSF), an inhibitor that contains a nitrile vibrational reporter. The nitrile stretch vibration of the water-soluble inhibitor model, potassium 4-cyanobenzenesulfonate (KCBSO), and the inhibitor were calibrated by IR solvent studies in H2O/DMSO and the frequency-temperature line-slope (FTLS) method in H2O and THF. The inhibitor complexes were examined by FTLS and the slopes of the best fit lines for subtilisin-CBS and trypsin-CBS in aqueous buffer were both measured to be -3.5×10-2 cm-1/°C. These slopes were intermediate in value between that of KCBSO in aqueous buffer and CBSF in THF, which suggests that the active-site nitriles in both proteases are mostly solvated. The X-ray crystal structures of the subtilisin-CBS and trypsin-CBS complexes were solved at 1.27 and 1.32 Å, respectively. The inhibitor was modelled in two conformations in subtilisin-CBS and in one conformation in the trypsin-CBS. The crystallographic data support the FTLS data that the active-site nitrile groups are mostly solvated and participate in hydrogen bonds with water molecules. The combination of IR spectroscopy utilizing vibrational reporters paired with X-ray crystallography provides a powerful approach to studying protein structure.
Keywords: crystallography; proteases; spectroscopy; vibrational reporters.
Figures






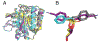
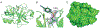


Similar articles
-
Exploring local solvation environments of a heme protein using the spectroscopic reporter 4-cyano-l-phenylalanine.RSC Adv. 2018 Apr 9;8(24):13503-13512. doi: 10.1039/c8ra02000k. Epub 2018 Apr 10. RSC Adv. 2018. PMID: 29780583 Free PMC article.
-
Receptor rigidity and ligand mobility in trypsin-ligand complexes.Proteins. 2005 Feb 1;58(2):407-17. doi: 10.1002/prot.20326. Proteins. 2005. PMID: 15578663
-
Quantifying the Effects of Hydrogen Bonding on Nitrile Frequencies in GFP: Beyond Solvent Exposure.J Phys Chem B. 2018 Jul 5;122(26):6733-6743. doi: 10.1021/acs.jpcb.8b03907. Epub 2018 Jun 25. J Phys Chem B. 2018. PMID: 29874077
-
Molecular recognition at the active site of subtilisin BPN': crystallographic studies using genetically engineered proteinaceous inhibitor SSI (Streptomyces subtilisin inhibitor).Protein Eng. 1991 Jun;4(5):501-8. doi: 10.1093/protein/4.5.501. Protein Eng. 1991. PMID: 1891457
-
Nitrile groups as vibrational probes of biomolecular structure and dynamics: an overview.Phys Chem Chem Phys. 2009 Oct 1;11(37):8119-32. doi: 10.1039/b908588b. Epub 2009 Jul 31. Phys Chem Chem Phys. 2009. PMID: 19756266 Review.
References
-
- Puente XS, Sánchez LM, Overall CM, López-Otín C, Nat. Rev. Genet 2003, 4, 544–558. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
