Safety and Efficacy of Leadless Pacemakers: A Systematic Review and Meta-Analysis
- PMID: 34169736
- PMCID: PMC8403316
- DOI: 10.1161/JAHA.120.019212
Safety and Efficacy of Leadless Pacemakers: A Systematic Review and Meta-Analysis
Abstract
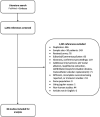
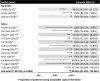
Background Leadless pacemaker is a novel technology, and evidence supporting its use is uncertain. We performed a systematic review and meta-analysis to examine the safety and efficacy of leadless pacemakers implanted in the right ventricle. Methods and Results We searched PubMed and Embase for studies published before June 6, 2020. The primary safety outcome was major complications, whereas the primary efficacy end point was acceptable pacing capture threshold (≤2 V). Pooled estimates were calculated using the Freedman-Tukey double arcsine transformation. Of 1281 records screened, we identified 36 observational studies of Nanostim and Micra leadless pacemakers, with most (69.4%) reporting outcomes for the Micra. For Micra, the pooled incidence of complications at 90 days (n=1608) was 0.46% (95% CI, 0.08%-1.05%) and at 1 year (n=3194) was 1.77% (95% CI, 0.76%-3.07%). In 5 studies with up to 1-year follow-up, Micra was associated with 51% lower odds of complications compared with transvenous pacemakers (3.30% versus 7.43%; odds ratio [OR], 0.49; 95% CI, 0.34-0.70). At 1 year, 98.96% (95% CI, 97.26%-99.94%) of 1376 patients implanted with Micra had good pacing capture thresholds. For Nanostim, the reported complication incidence ranged from 6.06% to 23.54% at 90 days and 5.33% to 6.67% at 1 year, with 90% to 100% having good pacing capture thresholds at 1 year (pooled result not estimated because of the low number of studies). Conclusions Most studies report outcomes for the Micra, which is associated with a low risk of complications and good electrical performance up to 1-year after implantation. Further data from randomized controlled trials are needed to support the widespread adoption of these devices in clinical practice.
Keywords: efficacy; leadless pacemaker; meta‐analysis; safety; systematic review.
Conflict of interest statement
Dr Denman has delivered talks for Medtronic on LPs, has run 4 training courses for Medtronic at The Prince Charles Hospital to train other physicians in how to implant the Micra LP. Dr Denman is also a local principal investigator for the St Jude Nanostim study. Dr Haqqani has received speaking and proctoring honoraria from Medtronic and has served on the scientific advisory board of Medtronic. Dr Haqqani has also received speaking honoraria from Abbott. The remaining authors have no disclosures to report.
Figures


References
-
- Kusumoto Fred M, Schoenfeld Mark H, Barrett C, Edgerton James R, Ellenbogen Kenneth A, Gold Michael R, Goldschlager NF, Hamilton RM, Joglar JA, Kim RJ, et al. 2018 ACC/AHA/HRS guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2019;140:e382–e482. - PubMed
-
- Ranasinghe I, Labrosciano C, Horton D, Ganesan A, Curtis JP, Krumholz HM, McGavigan A, Hossain S, Air T, Hariharaputhiran S, et al. Institutional variation in quality of cardiovascular implantable electronic device implantation: a cohort study. Ann Intern Med. 2019;171:309–317. DOI: 10.7326/M18-2810. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

