Cognizance of posttranslational modifications in vaccines: A way to enhanced immunogenicity
- PMID: 34170014
- PMCID: PMC8427110
- DOI: 10.1002/jcp.30483
Cognizance of posttranslational modifications in vaccines: A way to enhanced immunogenicity
Abstract
Vaccination is a significant advancement or preventative strategy for controlling the spread of various severe infectious and noninfectious diseases. The purpose of vaccination is to stimulate or activate the immune system by injecting antigens, i.e., either whole microorganisms or using the pathogen's antigenic part or macromolecules. Over time, researchers have made tremendous efforts to reduce vaccine side effects or failure by developing different strategies combining with immunoinformatic and molecular biology. These newly designed vaccines are composed of single or several antigenic molecules derived from a pathogenic organism. Although, whole-cell vaccines are still in use against various diseases but due to their ineffectiveness, other vaccines like DNA-based, RNA-based, and protein-based vaccines, with the addition of immunostimulatory agents, are in the limelight. Despite this, many researchers escape the most common fundamental phenomenon of protein posttranslational modifications during the development of vaccines, which regulates protein functional behavior, evokes immunogenicity and stability, etc. The negligence about post translational modification (PTM) during vaccine development may affect the vaccine's efficacy and immune responses. Therefore, it becomes imperative to consider these modifications of macromolecules before finalizing the antigenic vaccine construct. Here, we have discussed different types of posttranslational/transcriptional modifications that are usually considered during vaccine construct designing: Glycosylation, Acetylation, Sulfation, Methylation, Amidation, SUMOylation, Ubiquitylation, Lipidation, Formylation, and Phosphorylation. Based on the available research information, we firmly believe that considering these modifications will generate a potential and highly immunogenic antigenic molecule against communicable and noncommunicable diseases compared to the unmodified macromolecules.
Keywords: immunogenicity; infectious diseases; noninfectious diseases; posttranslational modifications; vaccines.
© 2021 Wiley Periodicals LLC.
Conflict of interest statement
The authors declare that there are no conflict of interests.
Figures
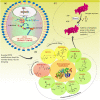
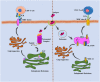
References
-
- Abdel‐Aal, A.‐B. M. , El‐Naggar, D. , Zaman, M. , Batzloff, M. , & Toth, I. (2012). Design of fully synthetic, self‐adjuvanting vaccine incorporating the tumor‐associated carbohydrate Tn antigen and lipoamino acid‐based Toll‐like Receptor 2 Ligand. Journal of Medicinal Chemistry, 55(15), 6968–6974. - PubMed
-
- Abdulhaqq, S. A. , & Weiner, D. B. (2008). DNA vaccines: Developing new strategies to enhance immune responses. Immunologic Research, 42(1‐3), 219–232. - PubMed
-
- Andersson, H. A. , & Barry, M. A. (2004). Maximizing antigen targeting to the proteasome for gene‐based vaccines. Molecular Therapy, 10(3), 432–446. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

