Selection and identification of single-domain antibody against Peste des Petits Ruminants virus
- PMID: 34170088
- PMCID: PMC8318796
- DOI: 10.4142/jvs.2021.22.e45
Selection and identification of single-domain antibody against Peste des Petits Ruminants virus
Abstract
Background: Peste des petits ruminants (PPR) is an infectious disease caused by the peste des petits ruminants virus (PPRV) that mainly produces respiratory symptoms in affected animals, resulting in great losses in the world's agriculture industry every year. Single-domain variable heavy chain (VHH) antibody fragments, also referred to as nanobodies, have high expression yields and other advantages including ease of purification and high solubility.
Objectives: The purpose of this study is to obtain a single-domain antibody with good reactivity and high specificity against PPRV.
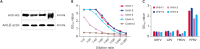
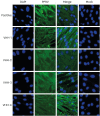
Methods: A VHH cDNA library was established by immunizing camels with PPRV vaccine, and the capacity and diversity of the library were examined. Four PPRV VHHs were selected, and the biological activity and antigen-binding capacity of the four VHHs were identified by western blot, indirect immunofluorescence, and enzyme-linked immunosorbent assay (ELISA) analyses. ELISA was used to identify whether the four VHHs were specific for PPRV, and VHH neutralization tests were carried out. ELISA and western blot analyses were used to identify which PPRV protein was targeted by VHH2.
Results: The PPRV cDNA library was constructed successfully. The library capacity was greater than 2.0 × 10⁶ cfu/mL, and the inserted fragment size was approximately 400 bp to 2000 bp. The average length of the cDNA library fragment was about 1000 bp, and the recombination rate was approximately 100%. Four single-domain antibody sequences were selected, and proteins expressed in the supernatant were obtained. The four VHHs were shown to have biological activity, close affinity to PPRV, and no cross-reaction with common sheep diseases. All four VHHs had neutralization activity, and VHH2 was specific to the PPRV M protein.
Conclusions: The results of this preliminary research of PPRV VHHs showed that four screened VHH antibodies could be useful in future applications. This study provided new materials for inclusion in PPRV research.
Keywords: Peste des petits ruminants virus; cDNA library; single-domain antibody.
© 2021 The Korean Society of Veterinary Science.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Immune responses in mice vaccinated with a suicidal DNA vaccine expressing the hemagglutinin glycoprotein from the peste des petits ruminants virus.J Virol Methods. 2013 Nov;193(2):525-30. doi: 10.1016/j.jviromet.2013.07.031. Epub 2013 Jul 27. J Virol Methods. 2013. PMID: 23896018
-
Rescue of recombinant peste des petits ruminants virus: creation of a GFP-expressing virus and application in rapid virus neutralization test.Vet Res. 2012 Jun 2;43(1):48. doi: 10.1186/1297-9716-43-48. Vet Res. 2012. PMID: 22658079 Free PMC article.
-
Serological evidence of camel exposure to peste des petits ruminants virus (PPRV) in Nigeria.Trop Anim Health Prod. 2015 Mar;47(3):603-6. doi: 10.1007/s11250-014-0747-6. Epub 2014 Dec 30. Trop Anim Health Prod. 2015. PMID: 25547805
-
Control of peste des petits ruminants: classical and new generation vaccines.Dev Biol (Basel). 2003;114:113-9. Dev Biol (Basel). 2003. PMID: 14677682 Review.
-
Peste des petits ruminants in large ruminants, camels and unusual hosts.Vet Q. 2020 Dec;40(1):35-42. doi: 10.1080/01652176.2020.1714096. Vet Q. 2020. PMID: 31917649 Free PMC article. Review.
Cited by
-
Development and characterization of a novel nanobody with SRMV neutralizing activity.Microb Cell Fact. 2024 Feb 10;23(1):45. doi: 10.1186/s12934-024-02311-6. Microb Cell Fact. 2024. PMID: 38341572 Free PMC article.
References
-
- Liu F, Li J, Li L, Liu Y, Wu X, Wang Z. Peste des petits ruminants in China since its first outbreak in 2007: A 10-year review. Transbound Emerg Dis. 2018;65(3):638–648. - PubMed
-
- Banyard AC, Parida S, Batten C, Oura C, Kwiatek O, Libeau G. Global distribution of peste des petits ruminants virus and prospects for improved diagnosis and control. J Gen Virol. 2010;91(Pt 12):2885–2897. - PubMed
-
- Wang D, Yang S, Yin S, Shang Y, Du P, Guo J, et al. Characterization of single-domain antibodies against Foot and Mouth Disease Virus (FMDV) serotype O from a camelid and imaging of FMDV in baby hamster kidney-21 cells with single-domain antibody-quantum dots probes. BMC Vet Res. 2015;11(1):120. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

