COVID-19 symptoms at hospital admission vary with age and sex: results from the ISARIC prospective multinational observational study
- PMID: 34170486
- PMCID: PMC8231091
- DOI: 10.1007/s15010-021-01599-5
COVID-19 symptoms at hospital admission vary with age and sex: results from the ISARIC prospective multinational observational study
Abstract
Background: The ISARIC prospective multinational observational study is the largest cohort of hospitalized patients with COVID-19. We present relationships of age, sex, and nationality to presenting symptoms.
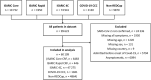
Methods: International, prospective observational study of 60 109 hospitalized symptomatic patients with laboratory-confirmed COVID-19 recruited from 43 countries between 30 January and 3 August 2020. Logistic regression was performed to evaluate relationships of age and sex to published COVID-19 case definitions and the most commonly reported symptoms.
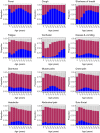
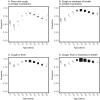
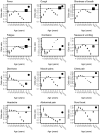
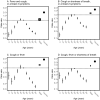
Results: 'Typical' symptoms of fever (69%), cough (68%) and shortness of breath (66%) were the most commonly reported. 92% of patients experienced at least one of these. Prevalence of typical symptoms was greatest in 30- to 60-year-olds (respectively 80, 79, 69%; at least one 95%). They were reported less frequently in children (≤ 18 years: 69, 48, 23; 85%), older adults (≥ 70 years: 61, 62, 65; 90%), and women (66, 66, 64; 90%; vs. men 71, 70, 67; 93%, each P < 0.001). The most common atypical presentations under 60 years of age were nausea and vomiting and abdominal pain, and over 60 years was confusion. Regression models showed significant differences in symptoms with sex, age and country.
Interpretation: This international collaboration has allowed us to report reliable symptom data from the largest cohort of patients admitted to hospital with COVID-19. Adults over 60 and children admitted to hospital with COVID-19 are less likely to present with typical symptoms. Nausea and vomiting are common atypical presentations under 30 years. Confusion is a frequent atypical presentation of COVID-19 in adults over 60 years. Women are less likely to experience typical symptoms than men.
Keywords: COVID-19; Case definition; Diagnosis; SARS-CoV-2; Symptoms.
© 2021. The Author(s).
Conflict of interest statement
M. Cheng declares grants from McGill Interdisciplinary Initiative in Infection and Immunity, and Canadian Institutes of Health Research; and personal fees from GEn1E Lifesciences (as a member of the scientific advisory board) and nplex biosciences (as a member of the scientific advisory board); M. Cummings and M. O'Donnell participated as investigators for completed and ongoing clinical trials evaluating the efficacy and safety of remdesivir (sponsored by Gilead Sciences) and convalescent plasma (sponsored by Amazon), in hospitalized patients with COVID-19—support for this work is paid to Columbia University; J. C. Holter declared grants from Research Council of Norway [grant 312780], and Vivaldi Invest A/S owned by Jon Stephenson von Tetzchner, during the conduct of the study; A.Kimmoun declared personal fees (payment for lectures) from Baxter, Aguettant, Aspen; D. Kumar declared grants and personal fees from Roche, GSK and Merck, and personal fees from Pfizer and Sanofi; F.X. Lescure declared personal fees (payment for lectures) from Gilead, MSD; and travel/accommodation/meeting expenses from Astellas, Eumedica, MSD; A. Pesenti declared personal fees from Maquet, Novalung/Xenios, Baxter, and Boehringer Ingelheim; S. Shrapnel reported grants from Prince Charles Hospital Foundation during the conduct of the study, and concurrently performed data analytics for the COVID-19 Critical Care Consortium; R. Tedder reports grants from MRC/UKRI during the conduct of the study, and has a patent United Kingdom Patent Application No. 2014047.1 “SARS-CoV-2 antibody detection assay” issued; J. Troost declared personal fees from General Electric and Procter and Gamble.
Figures
Update of
-
COVID-19 symptoms at hospital admission vary with age and sex: ISARIC multinational study.medRxiv [Preprint]. 2020 Nov 19:2020.10.26.20219519. doi: 10.1101/2020.10.26.20219519. medRxiv. 2020. Update in: Infection. 2021 Oct;49(5):889-905. doi: 10.1007/s15010-021-01599-5. PMID: 33140062 Free PMC article. Updated. Preprint.
References
-
- Struyf T, Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Leeflang MM, et al. Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19 disease. Cochrane Database Syst Rev. 2020;7(7):Cd013665. doi: 10.1002/14651858.Cd013665. - DOI - PMC - PubMed
-
- Liang WH, Guan WJ, Li CC, Li YM, Liang HR, Zhao Y, et al. Clinical characteristics and outcomes of hospitalised patients with COVID-19 treated in Hubei (epicentre) and outside Hubei (non-epicentre): a nationwide analysis of China. Eur Respir J. 2020;55(6):2000562. doi: 10.1183/13993003.00562-2020. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- MC_UU_12014/8/MRC_/Medical Research Council/United Kingdom
- 200927/WT_/Wellcome Trust/United Kingdom
- MR/S032304/1/MRC_/Medical Research Council/United Kingdom
- 204904/Z/16/Z/WT_/Wellcome Trust/United Kingdom
- MR/R015600/1/MRC_/Medical Research Council/United Kingdom
- MC_PC_19026/MRC_/Medical Research Council/United Kingdom
- UL1 TR002240/TR/NCATS NIH HHS/United States
- L30 HL159698/HL/NHLBI NIH HHS/United States
- 109965/Z/15/Z/WT_/Wellcome Trust/United Kingdom
- MC_PC_19012/MRC_/Medical Research Council/United Kingdom
- MC_PC_19059/MRC_/Medical Research Council/United Kingdom
- MC_PC_19025/MRC_/Medical Research Council/United Kingdom
- T32 GM112596/GM/NIGMS NIH HHS/United States
- MC_PC_15001/MRC_/Medical Research Council/United Kingdom
- UL1TR002240/NH/NIH HHS/United States

