Thalamus and claustrum control parallel layer 1 circuits in retrosplenial cortex
- PMID: 34170817
- PMCID: PMC8233040
- DOI: 10.7554/eLife.62207
Thalamus and claustrum control parallel layer 1 circuits in retrosplenial cortex
Abstract
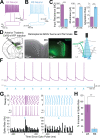
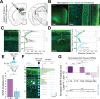
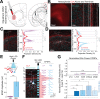
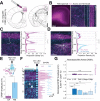
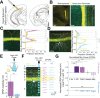
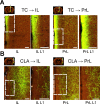
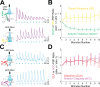
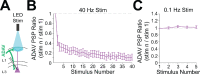
The granular retrosplenial cortex (RSG) is critical for both spatial and non-spatial behaviors, but the underlying neural codes remain poorly understood. Here, we use optogenetic circuit mapping in mice to reveal a double dissociation that allows parallel circuits in superficial RSG to process disparate inputs. The anterior thalamus and dorsal subiculum, sources of spatial information, strongly and selectively recruit small low-rheobase (LR) pyramidal cells in RSG. In contrast, neighboring regular-spiking (RS) cells are preferentially controlled by claustral and anterior cingulate inputs, sources of mostly non-spatial information. Precise sublaminar axonal and dendritic arborization within RSG layer 1, in particular, permits this parallel processing. Observed thalamocortical synaptic dynamics enable computational models of LR neurons to compute the speed of head rotation, despite receiving head direction inputs that do not explicitly encode speed. Thus, parallel input streams identify a distinct principal neuronal subtype ideally positioned to support spatial orientation computations in the RSG.
Keywords: angular head velocity; claustrum; head direction; mouse; neuroscience; retrosplenial cortex; spatial orientation; thalamus.
Plain language summary
Sitting in your car, about to drive home after a long day at work, you realize you have no idea which way to go: you recognize where you are right now, and you remember the name of the street your house is on, but you cannot figure out how to get there. This spatial disorientation happens to people with damage to a brain region called the retrosplenial cortex, whose role and inner workings remain poorly understood. Recent evidence has shown that this area contains ‘low-rheobase’ neurons which are not seen anywhere else in the brain, but what do these neurons do? Brennan, Jedrasiak-Cape, Kailasa et al. decided to explore the role of these neurons, focusing on the brain regions they are connected to. Experiments were conducted in mice using optogenetics, a technique that activates neurons using pulses of light. This revealed that brain areas involved in processing information about direction and position preferentially communicate with low-rheobase neurons rather than with nearby, more standard neurons in the retrosplenial cortex. The way these spatial signals are sent to the low-rheobase neurons allows these cells to ‘calculate’ how fast a mouse is turning its head using only information about which direction the mouse is facing. Essentially, this neuron can turn directional compass-like signals into a gyroscope signal that can track both direction and speed of head movement. These unique neurons may therefore be ideally suited to combine information about direction and space, suggesting that they may have evolved specifically to support spatial navigation. Individuals with Alzheimer’s disease show exactly the same type of spatial disorientation as individuals with direct damage to the retrosplenial cortex. This region is also one of the first to show altered activity in Alzheimer’s disease. Exploring whether these unique retrosplenial neurons and their communication patterns are altered in Alzheimer’s disease models could help to understand and potentially treat this debilitating condition.
© 2021, Brennan et al.
Conflict of interest statement
EB, IJ, SK, SR, SS, OA No competing interests declared
Figures






Similar articles
-
Representation of visual landmarks in retrosplenial cortex.Elife. 2020 Mar 10;9:e51458. doi: 10.7554/eLife.51458. Elife. 2020. PMID: 32154781 Free PMC article.
-
Cell-type-specific cholinergic control of granular retrosplenial cortex with implications for angular velocity coding across brain states.Prog Neurobiol. 2025 Aug;251:102804. doi: 10.1016/j.pneurobio.2025.102804. Epub 2025 Jul 8. Prog Neurobiol. 2025. PMID: 40639485 Free PMC article.
-
Hyperexcitable Neurons Enable Precise and Persistent Information Encoding in the Superficial Retrosplenial Cortex.Cell Rep. 2020 Feb 4;30(5):1598-1612.e8. doi: 10.1016/j.celrep.2019.12.093. Cell Rep. 2020. PMID: 32023472
-
Thalamocortical processing of the head-direction sense.Prog Neurobiol. 2019 Dec;183:101693. doi: 10.1016/j.pneurobio.2019.101693. Epub 2019 Sep 21. Prog Neurobiol. 2019. PMID: 31550513 Review.
-
Understanding retrosplenial amnesia: insights from animal studies.Neuropsychologia. 2010 Jul;48(8):2328-38. doi: 10.1016/j.neuropsychologia.2009.09.030. Epub 2009 Oct 2. Neuropsychologia. 2010. PMID: 19800900 Review.
Cited by
-
Changing the Cortical Conductor's Tempo: Neuromodulation of the Claustrum.Front Neural Circuits. 2021 May 13;15:658228. doi: 10.3389/fncir.2021.658228. eCollection 2021. Front Neural Circuits. 2021. PMID: 34054437 Free PMC article. Review.
-
Fine-Regional Role of the Claustrum in Anxiety and Higher Sensitivity to Cocaine in Adolescent Cocaine-Exposed Male Mice during Adulthood.J Neurosci. 2024 Jan 31;44(5):e0884232023. doi: 10.1523/JNEUROSCI.0884-23.2023. J Neurosci. 2024. PMID: 38148153 Free PMC article.
-
Differential stability of task variable representations in retrosplenial cortex.Nat Commun. 2024 Aug 11;15(1):6872. doi: 10.1038/s41467-024-51227-7. Nat Commun. 2024. PMID: 39127731 Free PMC article.
-
A Distinct Down-to-Up Transition Assembly in the Retrosplenial Cortex during Slow-Wave Sleep.J Neurosci. 2025 Apr 2;45(14):e1484242025. doi: 10.1523/JNEUROSCI.1484-24.2025. J Neurosci. 2025. PMID: 39952672
-
Regional and cell-type-specific afferent and efferent projections of the mouse claustrum.Cell Rep. 2023 Feb 28;42(2):112118. doi: 10.1016/j.celrep.2023.112118. Epub 2023 Feb 13. Cell Rep. 2023. PMID: 36774552 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

