Evaluation of a package of continuum of care interventions for improved maternal, newborn, and child health outcomes and service coverage in Ghana: A cluster-randomized trial
- PMID: 34170904
- PMCID: PMC8232410
- DOI: 10.1371/journal.pmed.1003663
Evaluation of a package of continuum of care interventions for improved maternal, newborn, and child health outcomes and service coverage in Ghana: A cluster-randomized trial
Abstract
Background: In low- and middle-income countries (LMICs), the continuum of care (CoC) for maternal, newborn, and child health (MNCH) is not always complete. This study aimed to evaluate the effectiveness of an integrated package of CoC interventions on the CoC completion, morbidity, and mortality outcomes of woman-child pairs in Ghana.
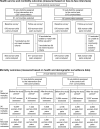
Methods and findings: This cluster-randomized controlled trial (ISRCTN: 90618993) was conducted at 3 Health and Demographic Surveillance System (HDSS) sites in Ghana. The primary outcome was CoC completion by a woman-child pair, defined as receiving antenatal care (ANC) 4 times or more, delivery assistance from a skilled birth attendant (SBA), and postnatal care (PNC) 3 times or more. Other outcomes were the morbidity and mortality of women and children. Women received a package of interventions and routine services at health facilities (October 2014 to December 2015). The package comprised providing a CoC card for women, CoC orientation for health workers, and offering women with 24-hour stay at a health facility or a home visit within 48 hours after delivery. In the control arm, women received routine services only. Eligibility criteria were as follows: women who gave birth or had a stillbirth from September 1, 2012 to September 30, 2014 (before the trial period), from October 1, 2014 to December 31, 2015 (during the trial period), or from January 1, 2016 to December 31, 2016 (after the trial period). Health service and morbidity outcomes were assessed before and during the trial periods through face-to-face interviews. Mortality was assessed using demographic surveillance data for the 3 periods above. Mixed-effects logistic regression models were used to evaluate the effectiveness as difference in differences (DiD). For health service and morbidity outcomes, 2,970 woman-child pairs were assessed: 1,480 from the baseline survey and 1,490 from the follow-up survey. Additionally, 33,819 cases were assessed for perinatal mortality, 33,322 for neonatal mortality, and 39,205 for maternal mortality. The intervention arm had higher proportions of completed CoC (410/870 [47.1%]) than the control arm (246/620 [39.7%]; adjusted odds ratio [AOR] for DiD = 1.77; 95% confidence interval [CI]: 1.08 to 2.92; p = 0.024). Maternal complications that required hospitalization during pregnancy were lower in the intervention (95/870 [10.9%]) than in the control arm (83/620 [13.4%]) (AOR for DiD = 0.49; 95% CI: 0.29 to 0.83; p = 0.008). Maternal mortality was 8/6,163 live births (intervention arm) and 4/4,068 live births during the trial period (AOR for DiD = 1.60; 95% CI: 0.40 to 6.34; p = 0.507) and 1/4,626 (intervention arm) and 9/3,937 (control arm) after the trial period (AOR for DiD = 0.11; 95% CI: 0.11 to 1.00; p = 0.050). Perinatal and neonatal mortality was not significantly reduced. As this study was conducted in a real-world setting, possible limitations included differences in the type and scale of health facilities and the size of subdistricts, contamination for intervention effectiveness due to the geographic proximity of the arms, and insufficient number of cases for the mortality assessment.
Conclusions: This study found that an integrated package of CoC interventions increased CoC completion and decreased maternal complications requiring hospitalization during pregnancy and maternal mortality after the trial period. It did not find evidence of reduced perinatal and neonatal mortality.
Trial registration: The study protocol was registered in the International Standard Randomised Controlled Trial Number Registry (90618993).
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- WHO, UNICEF, UNFPA, the World Bank, the United Nations Population Division. Trends in maternal mortality: 1990 to 2017. Geneva, Switzerland: World Health Organization; 2019.
-
- The United Nations Inter-agency Group for Child Mortality Estimation. Levels and trends in child mortality report 2019. New York, USA: United Nations Children’s Fund; 2019.
-
- Alkema L, Chou D, Hogan D, Zhang S, Moller AB, Gemmill A, et al.. Global, regional, and national levels and trends in maternal mortality between 1990 and 2015, with scenario-based projections to 2030: A systematic analysis by the UN Maternal Mortality Estimation Inter-Agency Group. Lancet. 2016;387(10017):462–74. doi: 10.1016/S0140-6736(15)00838-7 - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

