DRUL for school: Opening Pre-K with safe, simple, sensitive saliva testing for SARS-CoV-2
- PMID: 34170927
- PMCID: PMC8232451
- DOI: 10.1371/journal.pone.0252949
DRUL for school: Opening Pre-K with safe, simple, sensitive saliva testing for SARS-CoV-2
Abstract
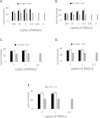
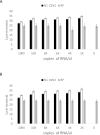
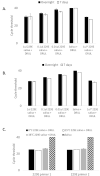
To address the need for simple, safe, sensitive, and scalable SARS-CoV-2 tests, we validated and implemented a PCR test that uses a saliva collection kit use at home. Individuals self-collected 300 μl saliva in vials containing Darnell Rockefeller University Laboratory (DRUL) buffer and extracted RNA was assayed by RT-PCR (the DRUL saliva assay). The limit of detection was confirmed to be 1 viral copy/μl in 20 of 20 replicate extractions. Viral RNA was stable in DRUL buffer at room temperature up to seven days after sample collection, and safety studies demonstrated that DRUL buffer immediately inactivated virus at concentrations up to 2.75x106 PFU/ml. Results from SARS-CoV-2 positive nasopharyngeal (NP) swab samples collected in viral transport media and assayed with a standard FDA Emergency Use Authorization (EUA) test were highly correlated with samples placed in DRUL buffer. Direct comparison of results from 162 individuals tested by FDA EUA oropharyngeal (OP) or NP swabs with co-collected saliva samples identified four otherwise unidentified positive cases in DRUL buffer. Over six months, we collected 3,724 samples from individuals ranging from 3 months to 92 years of age. This included collecting weekly samples over 10 weeks from teachers, children, and parents from a pre-school program, which allowed its safe reopening while at-risk pods were quarantined. In sum, we validated a simple, sensitive, stable, and safe PCR-based test using a self-collected saliva sample as a valuable tool for clinical diagnosis and screening at workplaces and schools.
Conflict of interest statement
T.P.S. wishes to thank the Richard Lounsbery Foundation, the Elinor Schwartz Charitable Trust and the Danica Foundation for support. DEO is an inventor on a provisional unlicensed patent entitled “Method and System for RNA Isolation from Self-Collected and Small Volume Samples”, US 63/050,155. C.M.R acknowledges the generous support of the G. Harold and Leila Y. Mathers Charitable Foundation, the BAWD Foundation, and The Rockefeller University. R.B.D. discloses that he receives consulting fees as a Senior Visiting Fellow at MITRE Corporation, that he has started a charitable LLC, D4S Testing, to offer free DRUL saliva testing to NYC school children, and that he is an Investigator of the Howard Hughes Medical Institute. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures


References
-
- CDC. COVID-19 Cases, Deaths, and Trends in the US. 28 Mar 2020 [cited 22 Nov 2020]. Available: https://covid.cdc.gov/covid-data-tracker
-
- Institute for Health Metrics and Evaluation. COVID-19 Projections. [cited Nove 22, 2020]. Available: https://covid19.healthdata.org/
-
- CDC. Overview of testing for SARS-CoV-2 (COVID-19). 10 Dec 2020. [cited 18 Feb 2021]. Available: https://www.cdc.gov/coronavirus/2019-ncov/hcp/testing-overview.html
-
- Akst J. RNA Extraction Kits for COVID-19 Tests Are in Short Supply in US. 11 Mar 2020. [cited 22 Nov 2020]. Available: https://www.the-scientist.com/news-opinion/rna-extraction-kits-for-covid...
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

