The effect of human amnion epithelial cells on lung development and inflammation in preterm lambs exposed to antenatal inflammation
- PMID: 34170941
- PMCID: PMC8232434
- DOI: 10.1371/journal.pone.0253456
The effect of human amnion epithelial cells on lung development and inflammation in preterm lambs exposed to antenatal inflammation
Abstract
Background: Lung inflammation and impaired alveolarization are hallmarks of bronchopulmonary dysplasia (BPD). We hypothesize that human amnion epithelial cells (hAECs) are anti-inflammatory and reduce lung injury in preterm lambs born after antenatal exposure to inflammation.
Methods: Pregnant ewes received either intra-amniotic lipopolysaccharide (LPS, from E.coli 055:B5; 4mg) or saline (Sal) on day 126 of gestation. Lambs were delivered by cesarean section at 128 d gestation (term ~150 d). Lambs received intravenous hAECs (LPS/hAECs: n = 7; 30x106 cells) or equivalent volumes of saline (LPS/Sal, n = 10; or Sal/Sal, n = 9) immediately after birth. Respiratory support was gradually de-escalated, aimed at early weaning from mechanical ventilation towards unassisted respiration. Lung tissue was collected 1 week after birth. Lung morphology was assessed and mRNA levels for inflammatory mediators were measured.
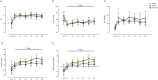
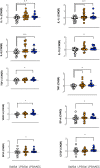
Results: Respiratory support required by LPS/hAEC lambs was not different to Sal/Sal or LPS/Sal lambs. Lung tissue:airspace ratio was lower in the LPS/Sal compared to Sal/Sal lambs (P<0.05), but not LPS/hAEC lambs. LPS/hAEC lambs tended to have increased septation in their lungs versus LPS/Sal (P = 0.08). Expression of inflammatory cytokines was highest in LPS/hAECs lambs.
Conclusions: Postnatal administration of a single dose of hAECs stimulates a pulmonary immune response without changing ventilator requirements in preterm lambs born after intrauterine inflammation.
Conflict of interest statement
The University of Western Australia (via JJP) has consultancy agreements with Chiesi Farmaceutici S.p.A. unrelated to the subject of this study. Fisher & Paykel Healthcare have material transfer agreements with Hudson Research Institute that are also unrelated to this work. There are no other relevant interests relating to employment, consultancy, patents, or products in development or marketed products to declare. These material transfer agreements do not alter our adherence to PLOS ONE policies on sharing data and materials. None of the authors have conflicts of interest to disclose.
Figures




References
-
- Viscardi RM, Muhumuza CK, Rodriguez A, Fairchild KD, Sun CC, Gross GW, et al. Inflammatory markers in intrauterine and fetal blood and cerebrospinal fluid compartments are associated with adverse pulmonary and neurologic outcomes in preterm infants. Pediatr Res. 2004;55(6):1009–17. doi: 10.1203/01.pdr.0000127015.60185.8a - DOI - PubMed
-
- Choi CW, Kim BI, Kim HS, Park JD, Choi JH, Son DW. Increase of interleukin-6 in tracheal aspirate at birth: a predictor of subsequent bronchopulmonary dysplasia in preterm infants. Acta Paediatr. 2006;95(1):38–43. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

