Improved outcomes over time for adult COVID-19 patients with acute respiratory distress syndrome or acute respiratory failure
- PMID: 34170950
- PMCID: PMC8232521
- DOI: 10.1371/journal.pone.0253767
Improved outcomes over time for adult COVID-19 patients with acute respiratory distress syndrome or acute respiratory failure
Abstract
Background: COVID-19's pulmonary manifestations are broad, ranging from pneumonia with no supplemental oxygen requirements to acute respiratory distress syndrome (ARDS) with acute respiratory failure (ARF). In response, new oxygenation strategies and therapeutics have been developed, but their large-scale effects on outcomes in severe COVID-19 patients remain unknown. Therefore, we aimed to examine the trends in mortality, mechanical ventilation, and cost over the first six months of the pandemic for adult COVID-19 patients in the US who developed ARDS or ARF.
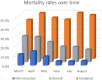
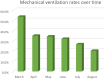
Methods and findings: The Vizient Clinical Data Base, a national database comprised of administrative, clinical, and financial data from academic medical centers, was queried for patients ≥ 18-years-old with COVID-19 and either ARDS or ARF admitted between 3/2020-8/2020. Demographics, mechanical ventilation, length of stay, total cost, mortality, and discharge status were collected. Mann-Kendall tests were used to assess for significant monotonic trends in total cost, mechanical ventilation, and mortality over time. Chi-square tests were used to compare mortality rates between March-May and June-August. 110,223 adult patients with COVID-19 ARDS or ARF were identified. Mean length of stay was 12.1±13.3 days and mean total cost was $35,991±32,496. Mechanical ventilation rates were 34.1% and in-hospital mortality was 22.5%. Mean cost trended downward over time (p = 0.02) from $55,275 (March) to $18,211 (August). Mechanical ventilation rates trended down (p<0.01) from 53.8% (March) to 20.3% (August). Overall mortality rates also decreased (p<0.01) from 28.4% (March) to 13.7% (August). Mortality rates in mechanically ventilated patients were similar over time (p = 0.45), but mortality in patients not requiring mechanical ventilation decreased from March-May compared to June-July (13.5% vs 4.6%, p<0.01).
Conclusions: This study describes the outcomes of a large cohort with COVID-19 ARDS or ARF and the subsequent decrease in cost, mechanical ventilation, and mortality over the first 6 months of the pandemic in the US.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: Alpesh Amin reported serving as PI or co-I of clinical trials sponsored by NIH/NIAID, NeuroRx Pharma, Pulmotect, Blade Therpeutics, Novartis, Takeda, Humanigen, Eli Lilly, PTC Therapeutics, OctaPharma, Fulcrum Therapeutics, Alexion. He has served as speaker and/or consultant for BMS, Pfizer, BI, Portola, Sunovion, Mylan, Salix, Alexion, AstraZeneca, Novartis, Nabriva, Paratek, Bayer, Tetraphase, Achogen LaJolla, Millenium, HeartRite, Aseptiscope, Sprightly. Ninh Nguyen reported serving as a speaker for Olympus and Endogastric Solutions. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
References
-
- Lai CC, Liu YH, Wang CY, Wang YH, Hsueh SC, Yen MY, et al. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): Facts and myths. J Microbiol Immunol Infect. 2020. Jun;53(3):404–412. doi: 10.1016/j.jmii.2020.02.012 - DOI - PMC - PubMed

