Histopathological and immunohistochemical aspects of bone tissue in aseptic necrosis of the femoral head
- PMID: 34171073
- PMCID: PMC8343594
- DOI: 10.47162/RJME.61.4.26
Histopathological and immunohistochemical aspects of bone tissue in aseptic necrosis of the femoral head
Abstract
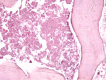
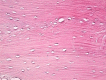
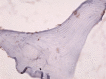
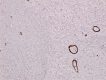
Femoral head osteonecrosis, also known as avascular necrosis, is a disease with a multifactorial etiology, characterized by a profound change of bone architecture, which leads to the diminishing of bone resistance and femoral head collapse. The main causes that lead to femoral head necrosis are represented by the decrease of local blood perfusion and increase of intraosseous pressure, because of an excessive development of adipose tissue in the areolas of the trabecular bone tissue in the femoral head. The histopathological and immunohistochemical (IHC) study performed by us showed that most of bone trabeculae were damaged by necrotic-involutive processes, their sizes being reduced, both regarding their length and their diameter; generally, the spans were thin, fragmented, distanced among them, which led to the occurrence of some large areolar cavities, full of conjunctive tissue, rich in adipocytes. Some of the residual bone spans even presented microfractures. In the structure of the trabecular bone tissue, numerous cavities showed lack of content, which indicates the death of osteocytes inside, while the endosteum appeared very thin, with few osteoprogenitor, flattened, difficult to highlight cells. The IHC study showed a low reaction of the bone reparatory processes and a reduced multiplication capacity of bone cells involved in the remodeling and remake of the diseased bone tissue. Nevertheless, there were identified numerous young conjunctive cells (fibroblasts, myofibroblasts), positive to proliferating cell nuclear antigen (PCNA), cells that have a high capacity of multiplication, participating in the formation of a fibrous conjunctive tissue (sclerous) instead of the damaged bone trabeculae. The formation of fibrous conjunctive tissue causes the reduction of mechanical resistance of the femoral head and its collapse. The IHC study of the microvascularization in the femoral head damaged by aseptic osteonecrosis showed the presence of a very low vascular system, both in the residual bone trabeculae and in the sclerous conjunctive tissue. Of the inflammatory cells present in the spongy bone tissue of the femoral head affected by osteonecrosis, the most numerous ones were the macrophages. Both macrophages and T- and B-lymphocytes had a heterogenous distribution.
Conflict of interest statement
The authors declare that they have no conflict of interests.
Figures















Similar articles
-
Analysis of early stage osteonecrosis of the human femoral head and the mechanism of femoral head collapse.Int J Biol Sci. 2018 Jan 14;14(2):156-164. doi: 10.7150/ijbs.18334. eCollection 2018. Int J Biol Sci. 2018. PMID: 29483834 Free PMC article.
-
Necrosis of the femoral head, X-ray microtomography (microCT) and histology of retrieved human femoral heads.Morphologie. 2021 Jun;105(349):134-142. doi: 10.1016/j.morpho.2021.02.008. Epub 2021 Mar 17. Morphologie. 2021. PMID: 33744124
-
[Aseptic necrosis of the femoral head in young adults].Int Orthop. 1984;8(2):77-88. doi: 10.1007/BF00265829. Int Orthop. 1984. PMID: 6386708 Review. French.
-
Experimentally gained insight - based proposal apropos the treatment of osteonecrosis of the femoral head.Med Hypotheses. 2004;62(6):958-65. doi: 10.1016/j.mehy.2003.12.036. Med Hypotheses. 2004. PMID: 15142657
-
Vasculature deprivation--induced osteonecrosis of the rat femoral head as a model for therapeutic trials.Theor Biol Med Model. 2005 Jul 5;2:24. doi: 10.1186/1742-4682-2-24. Theor Biol Med Model. 2005. PMID: 15996271 Free PMC article. Review.
Cited by
-
[Role and mechanism of macrophage-mediated osteoimmune in osteonecrosis of the femoral head].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2024 Jan 15;38(1):119-124. doi: 10.7507/1002-1892.202308026. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2024. PMID: 38225851 Free PMC article. Chinese.
-
Post-COVID-19 Femoral Head Osteonecrosis Exhibits Mast Cell Clusters, Fibrosis, and Vascular Thrombosis: Key Pathological Mechanisms in Long COVID-19 Bone Degeneration.Pathophysiology. 2025 Jul 18;32(3):36. doi: 10.3390/pathophysiology32030036. Pathophysiology. 2025. PMID: 40700078 Free PMC article.
-
The role of immune cells in modulating chronic inflammation and osteonecrosis.Front Immunol. 2022 Dec 13;13:1064245. doi: 10.3389/fimmu.2022.1064245. eCollection 2022. Front Immunol. 2022. PMID: 36582244 Free PMC article. Review.
-
The Role of Structural Deterioration and Biomechanical Changes of the Necrotic Lesion in Collapse Mechanism of Osteonecrosis of the Femoral Head.Orthop Surg. 2022 May;14(5):831-839. doi: 10.1111/os.13277. Epub 2022 Apr 21. Orthop Surg. 2022. PMID: 35445585 Free PMC article. Review.
-
Assessment of pain intensity after total hip arthroplasty using the Visual Analogue Scale (VAS).J Med Life. 2024 Dec;17(12):1049-1053. doi: 10.25122/jml-2024-0362. J Med Life. 2024. PMID: 39877038 Free PMC article.
References
-
- Amanatullah DF, Strauss EJ, Di Cesare PE. Current management options for osteonecrosis of the femoral head: Part II, operative management. Am J Orthop (Belle Mead NJ) 2011;40(10):E216–E225. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

