Analysis of the distribution and expression of some tumor invasiveness markers in palate squamous cell carcinomas
- PMID: 34171074
- PMCID: PMC9016711
- DOI: 10.47162/RJME.61.4.27
Analysis of the distribution and expression of some tumor invasiveness markers in palate squamous cell carcinomas
Abstract
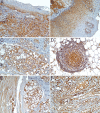
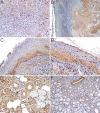
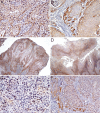
Oral cancer remains an important global health issue and despite recent diagnostic and therapeutic advances, it continues to have an unfavorable prognostic and decreased survival. Although palatal tumors represent one of the rarest locations of oral squamous cell carcinomas (SCCs), they are among the most aggressive local tumors, leaving behind important morpho-functional disabilities. In order to explain such local aggressiveness, the present study aims to investigate the immunohistochemical expression in palate SCCs of some markers known to be involved in the process of tumor invasiveness, such as Wiskott-Aldrich syndrome like (WASL), Claudin-1 (CLDN1), Integrin beta-6 (ITGB6) and c-Mesenchymal to epithelial transition protein (c-Met). We have found here a higher tumor WASL and CLDN1 reactivity in well-differentiated (G1) palate SCCs, and regardless the histological type, degree of differentiation or tumor topography, an overexpression at the invasion front, and in those palate' SCC cases with muscular invasiveness and with lymph node (LN) dissemination. ITGB6 and c-Met had a higher reactivity in moderately differentiated (G2) palate SCCs, especially at the periphery of tumor proliferations, at the invasion front and in those high invasive cases and as well as in those that associated LN dissemination. All four investigated markers were also positive at the level of LN metastatic proliferations. None of the markers could statistically stratify on age group and pain, and on bone and perineural invasion while all of them statistically stratified on survival and grading. We concluded that these markers have a prognostic role allowing the identification of those cases with an unfavorable clinical evolution and decreased survival.
Conflict of interest statement
The authors declare that there is no conflict of interests regarding the publication of this paper. All authors read and approved the final manuscript.
Figures








Similar articles
-
Immunohistochemical evaluation of D2-40, Galectin-3, Maspin and MCM7 expression in palate squamous cell carcinomas.Rom J Morphol Embryol. 2021 Jan-Mar;62(1):133-149. doi: 10.47162/RJME.62.1.13. Rom J Morphol Embryol. 2021. PMID: 34609416 Free PMC article.
-
Invasive Front Grading and Epithelial-Mesenchymal Transition in Canine Oral and Cutaneous Squamous Cell Carcinomas.Vet Pathol. 2017 Sep;54(5):783-791. doi: 10.1177/0300985817707005. Epub 2017 May 11. Vet Pathol. 2017. PMID: 28494700
-
The clinicopathological significances and biological functions of parafibromin expression in head and neck squamous cell carcinomas.Tumour Biol. 2015 Dec;36(12):9487-97. doi: 10.1007/s13277-015-3618-5. Epub 2015 Jul 1. Tumour Biol. 2015. PMID: 26124004
-
Vascular endothelial growth factor is a marker of tumor invasion and metastasis in squamous cell carcinomas of the head and neck.Clin Cancer Res. 1999 Apr;5(4):775-82. Clin Cancer Res. 1999. PMID: 10213212
-
Clear-cell differentiation and lymphatic invasion, but not the revised TNM classification, predict lymph node metastases in pT1 penile cancer: a clinicopathologic study of 76 patients from a low incidence area.Urol Oncol. 2013 Oct;31(7):1378-85. doi: 10.1016/j.urolonc.2012.01.017. Epub 2012 Mar 14. Urol Oncol. 2013. PMID: 22421354
Cited by
-
Immunohistochemical evaluation of D2-40, Galectin-3, Maspin and MCM7 expression in palate squamous cell carcinomas.Rom J Morphol Embryol. 2021 Jan-Mar;62(1):133-149. doi: 10.47162/RJME.62.1.13. Rom J Morphol Embryol. 2021. PMID: 34609416 Free PMC article.
-
Upregulation of SEMP1 Contributes to Improving the Biological Functions of Trophoblast via the PI3K/AKT Pathway in Preeclampsia.Mol Biotechnol. 2024 Mar;66(3):531-543. doi: 10.1007/s12033-023-00774-3. Epub 2023 Jun 5. Mol Biotechnol. 2024. PMID: 37277581
References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. - PubMed
-
- Omura K. Current status of oral cancer treatment strategies: surgical treatments for oral squamous cell carcinoma. Int J Clin Oncol. 2014;19(3):423–430. - PubMed
-
- Oskam IM, Verdonck-de Leeuw IM, Aaronson NK, Witte BI, de Bree R, Doornaert P, Langendijk JA, Leemans CR. Prospective evaluation of health-related quality of life in long-term oral and oropharyngeal cancer survivors and the perceived need for supportive care. Oral Oncol. 2013;49(5):443–448. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

