Acclimation of photosynthetic apparatus in the mesophilic red alga Dixoniella giordanoi
- PMID: 34171145
- PMCID: PMC8596783
- DOI: 10.1111/ppl.13489
Acclimation of photosynthetic apparatus in the mesophilic red alga Dixoniella giordanoi
Abstract
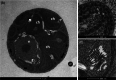
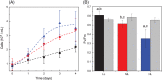
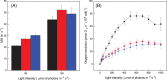
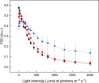
Eukaryotic algae are photosynthetic organisms capable of exploiting sunlight to fix carbon dioxide into biomass with highly variable genetic and metabolic features. Information on algae metabolism from different species is inhomogeneous and, while green algae are, in general, more characterized, information on red algae is relatively scarce despite their relevant position in eukaryotic algae diversity. Within red algae, the best-known species are extremophiles or multicellular, while information on mesophilic unicellular organisms is still lacunose. Here, we investigate the photosynthetic properties of a recently isolated seawater unicellular mesophilic red alga, Dixoniella giordanoi. Upon exposure to different illuminations, D. giordanoi shows the ability to acclimate, modulate chlorophyll content, and re-organize thylakoid membranes. Phycobilisome content is also largely regulated, leading to almost complete disassembly of this antenna system in cells grown under intense illumination. Despite the absence of a light-induced xanthophyll cycle, cells accumulate zeaxanthin upon prolonged exposure to strong light, likely contributing to photoprotection. D. giordanoi cells show the ability to perform cyclic electron transport that is enhanced under strong illumination, likely contributing to the protection of Photosystem I from over-reduction and enabling cells to survive PSII photoinhibition without negative impact on growth.
© 2021 The Authors. Physiologia Plantarum published by John Wiley & Sons Ltd on behalf of Scandinavian Plant Physiology Society.
Figures



Similar articles
-
Photoacclimation of photosynthesis in the Eustigmatophycean Nannochloropsis gaditana.Photosynth Res. 2016 Sep;129(3):291-305. doi: 10.1007/s11120-016-0297-z. Epub 2016 Jul 22. Photosynth Res. 2016. PMID: 27448115
-
Acclimation of Norway spruce photosynthetic apparatus to the combined effect of high irradiance and temperature.J Plant Physiol. 2010 May 15;167(8):597-605. doi: 10.1016/j.jplph.2009.11.011. Epub 2010 Jan 8. J Plant Physiol. 2010. PMID: 20060196
-
Extensive remodeling of the photosynthetic apparatus alters energy transfer among photosynthetic complexes when cyanobacteria acclimate to far-red light.Biochim Biophys Acta Bioenerg. 2020 Apr 1;1861(4):148064. doi: 10.1016/j.bbabio.2019.148064. Epub 2019 Aug 14. Biochim Biophys Acta Bioenerg. 2020. PMID: 31421078
-
Far-red light acclimation in diverse oxygenic photosynthetic organisms.Photosynth Res. 2019 Dec;142(3):349-359. doi: 10.1007/s11120-019-00653-6. Epub 2019 Jun 19. Photosynth Res. 2019. PMID: 31222688 Review.
-
Photoprotection of photosystems in fluctuating light intensities.J Exp Bot. 2015 May;66(9):2427-36. doi: 10.1093/jxb/eru463. Epub 2014 Dec 1. J Exp Bot. 2015. PMID: 25468932 Review.
Cited by
-
Growth and Photosynthetic Efficiency of Microalgae and Plants with Different Levels of Complexity Exposed to a Simulated M-Dwarf Starlight.Life (Basel). 2023 Jul 28;13(8):1641. doi: 10.3390/life13081641. Life (Basel). 2023. PMID: 37629498 Free PMC article.
-
Effects of Sulfate Limitation on Photosynthesis and Cell Composition of Unicellular Marine Microalgae of Different Phylogenies.Physiol Plant. 2025 Jul-Aug;177(4):e70401. doi: 10.1111/ppl.70401. Physiol Plant. 2025. PMID: 40673469 Free PMC article.
-
Regulation of Microalgal Photosynthetic Electron Transfer.Plants (Basel). 2024 Jul 29;13(15):2103. doi: 10.3390/plants13152103. Plants (Basel). 2024. PMID: 39124221 Free PMC article. Review.
-
Kovacikia euganea sp. nov. (Leptolyngbyaceae, Cyanobacteria), a new chlorophyll f producing cyanobacterium from the Euganean Thermal District (Italy).Front Microbiol. 2025 Mar 10;16:1545008. doi: 10.3389/fmicb.2025.1545008. eCollection 2025. Front Microbiol. 2025. PMID: 40130236 Free PMC article.
References
-
- Alboresi, A. , Storti, M. & Morosinotto, T. (2019) Balancing protection and efficiency in the regulation of photosynthetic electron transport across plant evolution. The New Phytologist, 221, 105–109. - PubMed
-
- Allahverdiyeva, Y. , Suorsa, M. , Tikkanen, M. & Aro, E.‐M.E.‐M. (2015) Photoprotection of photosystems in fluctuating light intensities. Journal of Experimental Botany, 66, 2427–2436. - PubMed
-
- Antoshvili, M. , Caspy, I. , Hippler, M. & Nelson, N. (2019) Structure and function of photosystem I in Cyanidioschyzon merolae. Photosynthesis Research, 139, 499–508. - PubMed
-
- Ballottari, M. , Dall'Osto, L. , Morosinotto, T. & Bassi, R. (2007) Contrasting behavior of higher plant photosystem I and II antenna systems during acclimation. The Journal of Biological Chemistry, 282, 8947–8958. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

