Iron preparations for women of reproductive age with iron deficiency anaemia in pregnancy (FRIDA): a systematic review and network meta-analysis
- PMID: 34171281
- PMCID: PMC7612251
- DOI: 10.1016/S2352-3026(21)00137-X
Iron preparations for women of reproductive age with iron deficiency anaemia in pregnancy (FRIDA): a systematic review and network meta-analysis
Abstract
Background: Numerous iron preparations are available for the treatment of iron deficiency anaemia in pregnancy. We aimed to provide a summary of the effectiveness and safety of iron preparations used in this setting.
Methods: We did a systematic review and network meta-analysis of randomised trials. We searched MEDLINE, Embase, Cochrane Central Register of Controlled Trials, trial registers, and grey literature for trials published in any language from Jan 1, 2011, to Feb 28, 2021. We included trials including pregnant women with iron deficiency anaemia and evaluating iron preparations, irrespective of administration route, with at least 60 mg of elemental iron, in comparison with another iron or non-iron preparation. Three authors independently selected studies, extracted data, and did a risk of bias assessment using the Cochrane tool (version 1.0). The primary outcome was the effectiveness of iron preparations, evaluated by changes in haemoglobin concentration at 4 weeks from baseline. The secondary outcomes were change in serum ferritin concentration at 4 weeks from baseline and treatment-related severe and non-severe adverse events. We did random-effects pairwise and network meta-analyses. Side-effects were reported descriptively for each trial. This study is registered with PROSPERO, CRD42018100822.
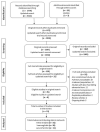
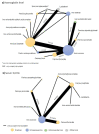
Findings: Among 3037 records screened, 128 full-text articles were further assessed for eligibility. Of the 53 eligible trials (reporting on 9145 women), 30 (15 interventions; 3243 women) contributed data to the network meta-analysis for haemoglobin and 15 (nine interventions; 1396 women) for serum ferritin. The risk of bias varied across the trials contributing to network meta-analysis, with 22 of 30 trials in the network meta-analysis for haemoglobin judged to have a high or medium global risk of bias. Compared with oral ferrous sulfate, intravenous iron sucrose improved both haemoglobin (mean difference 7·17 g/L, 95% CI 2·62-11·73; seven trials) and serum ferritin (mean difference 49·66 μg/L, 13·63-85·69; four trials), and intravenous ferric carboxymaltose improved haemoglobin (mean difference 8·52 g/L, 0·51-16·53; one trial). The evidence for other interventions compared with ferrous sulfate was insufficient. The most common side-effects with oral iron preparations were gastrointestinal effects (nausea, vomiting, and altered bowel movements). Side-effects were less common with parenteral iron preparations, although these included local pain, skin irratation, and, on rare occasions, allergic reactions.
Interpretation: Iron preparations for treatment of iron deficiency anaemia in pregnancy vary in effectiveness, with good evidence of benefit for intravenous iron sucrose and some evidence for intravenous ferric carboxymaltose. Clinicians and policy makers should consider the effectiveness of individual preparations before administration, to ensure effective treatment.
Funding: None.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests JD was a member of an advisory panel assessing the side-effect profiles of intravenous iron preparations for Pharmacosmos in 2018. All other authors declare no competing interests.
Figures
Similar articles
-
Fortification of salt with iron and iodine versus fortification of salt with iodine alone for improving iron and iodine status.Cochrane Database Syst Rev. 2022 Apr 21;4(4):CD013463. doi: 10.1002/14651858.CD013463.pub2. Cochrane Database Syst Rev. 2022. PMID: 35446435 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
Daily iron supplementation for improving anaemia, iron status and health in menstruating women.Cochrane Database Syst Rev. 2016 Apr 18;4(4):CD009747. doi: 10.1002/14651858.CD009747.pub2. Cochrane Database Syst Rev. 2016. PMID: 27087396 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
Cited by
-
Clinical outcome post treatment of anemia in pregnancy with intravenous versus oral iron therapy: a systematic review and meta-analysis.Sci Rep. 2024 Jan 2;14(1):179. doi: 10.1038/s41598-023-50234-w. Sci Rep. 2024. PMID: 38167523 Free PMC article.
-
Essentiality of Trace Elements in Pregnancy, Fertility, and Gynecologic Cancers-A State-of-the-Art Review.Nutrients. 2021 Dec 31;14(1):185. doi: 10.3390/nu14010185. Nutrients. 2021. PMID: 35011060 Free PMC article. Review.
-
Effect and safety of intravenous iron compared to oral iron for treatment of iron deficiency anaemia in pregnancy.Cochrane Database Syst Rev. 2024 Dec 9;12(12):CD016136. doi: 10.1002/14651858.CD016136. Cochrane Database Syst Rev. 2024. PMID: 39651609
-
Iron deficiency anaemia: pathophysiology, assessment, practical management.BMJ Open Gastroenterol. 2022 Jan;9(1):e000759. doi: 10.1136/bmjgast-2021-000759. BMJ Open Gastroenterol. 2022. PMID: 34996762 Free PMC article. Review.
-
Data integrity of 14 randomised controlled trials.Eur J Obstet Gynecol Reprod Biol. 2024 Jul;298:98-103. doi: 10.1016/j.ejogrb.2024.05.007. Epub 2024 May 10. Eur J Obstet Gynecol Reprod Biol. 2024. PMID: 38735122 Free PMC article.
References
-
- Ganz T. Systemic iron homeostasis. Physiological reviews. 2013;93(4):1721–41. - PubMed
-
- Barroso F, Allard S, Kahan BC, et al. Prevalence of maternal anaemia and its predictors: a multi-centre study. European journal of obstetrics, gynecology, and reproductive biology. 2011;159(1):99–105. - PubMed
-
- Daru J, Zamora J, Fernández-Félix BM, et al. Risk of maternal mortality in women with severe anaemia during pregnancy and post partum: a multilevel analysis. The Lancet Global Health. 2018;6(5):e548–e54. - PubMed
-
- Stevens GA, Finucane MM, De-Regil LM, et al. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995-2011: a systematic analysis of population-representative data. The Lancet Global health. 2013;1(1):e16–e25. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources