Controlling one's world: Identification of sub-regions of primate PFC underlying goal-directed behavior
- PMID: 34171290
- PMCID: PMC8346232
- DOI: 10.1016/j.neuron.2021.06.003
Controlling one's world: Identification of sub-regions of primate PFC underlying goal-directed behavior
Abstract
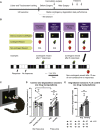
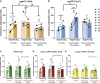
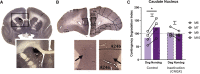
Impaired detection of causal relationships between actions and their outcomes can lead to maladaptive behavior. However, causal roles of specific prefrontal cortex (PFC) sub-regions and the caudate nucleus in mediating such relationships in primates are unclear. We inactivated and overactivated five PFC sub-regions, reversibly and pharmacologically: areas 24 (perigenual anterior cingulate cortex), 32 (medial PFC), 11 (anterior orbitofrontal cortex, OFC), 14 (rostral ventromedial PFC/medial OFC), and 14-25 (caudal ventromedial PFC) and the anteromedial caudate to examine their role in expressing learned action-outcome contingencies using a contingency degradation paradigm in marmoset monkeys. Area 24 or caudate inactivation impaired the response to contingency change, while area 11 inactivation enhanced it, and inactivation of areas 14, 32, or 14-25 had no effect. Overactivation of areas 11 and 24 impaired this response. These findings demonstrate the distinct roles of PFC sub-regions in goal-directed behavior and illuminate the candidate neurobehavioral substrates of psychiatric disorders, including obsessive-compulsive disorder.
Keywords: anterior cingulate cortex; caudate nucleus; common marmoset; contingency degradation; goal-directed behavior; habits; obsessive-compulsive disorder; orbitofrontal cortex; ventromedial prefrontal cortex.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


Comment in
-
If I do A, B will happen: Dissecting circuits detecting causal relations between actions and outcomes in marmoset prefrontal cortex.Neuron. 2021 Aug 4;109(15):2370-2373. doi: 10.1016/j.neuron.2021.07.012. Neuron. 2021. PMID: 34352212
Similar articles
-
Role of the Perigenual Anterior Cingulate and Orbitofrontal Cortex in Contingency Learning in the Marmoset.Cereb Cortex. 2016 Jul;26(7):3273-84. doi: 10.1093/cercor/bhw067. Epub 2016 Apr 29. Cereb Cortex. 2016. PMID: 27130662 Free PMC article.
-
Chemogenetic Disconnection between the Orbitofrontal Cortex and the Rostromedial Caudate Nucleus Disrupts Motivational Control of Goal-Directed Action.J Neurosci. 2022 Aug 10;42(32):6267-6275. doi: 10.1523/JNEUROSCI.0229-22.2022. Epub 2022 Jul 6. J Neurosci. 2022. PMID: 35794012 Free PMC article.
-
Lesions of the orbitofrontal but not medial prefrontal cortex disrupt conditioned reinforcement in primates.J Neurosci. 2003 Dec 3;23(35):11189-201. doi: 10.1523/JNEUROSCI.23-35-11189.2003. J Neurosci. 2003. PMID: 14657178 Free PMC article.
-
Divergent plasticity of prefrontal cortex networks.Neuropsychopharmacology. 2008 Jan;33(1):42-55. doi: 10.1038/sj.npp.1301554. Epub 2007 Oct 3. Neuropsychopharmacology. 2008. PMID: 17912252 Free PMC article. Review.
-
Comparing the prefrontal cortex of rats and primates: insights from electrophysiology.Neurotox Res. 2008 Oct;14(2-3):249-62. doi: 10.1007/BF03033814. Neurotox Res. 2008. PMID: 19073430 Review.
Cited by
-
Foraging with the frontal cortex: A cross-species evaluation of reward-guided behavior.Neuropsychopharmacology. 2022 Jan;47(1):134-146. doi: 10.1038/s41386-021-01140-0. Epub 2021 Aug 18. Neuropsychopharmacology. 2022. PMID: 34408279 Free PMC article. Review.
-
Adaptive Responding to Stimulus-Outcome Associations Requires Noradrenergic Transmission in the Medial Prefrontal Cortex.J Neurosci. 2024 May 29;44(22):e0078242024. doi: 10.1523/JNEUROSCI.0078-24.2024. J Neurosci. 2024. PMID: 38684363 Free PMC article.
-
A feasibility study of goal-directed network-based real-time fMRI neurofeedback for anhedonic depression.Front Psychiatry. 2023 Dec 5;14:1253727. doi: 10.3389/fpsyt.2023.1253727. eCollection 2023. Front Psychiatry. 2023. PMID: 38125285 Free PMC article.
-
Cortical glutamate and GABA are related to compulsive behaviour in individuals with obsessive compulsive disorder and healthy controls.Nat Commun. 2023 Jun 27;14(1):3324. doi: 10.1038/s41467-023-38695-z. Nat Commun. 2023. PMID: 37369695 Free PMC article.
-
Pyk2 Stabilizes Striatal Medium Spiny Neuron Structure and Striatal-Dependent Action.Cells. 2021 Dec 7;10(12):3442. doi: 10.3390/cells10123442. Cells. 2021. PMID: 34943950 Free PMC article.
References
-
- Anderson C.M., Swanson R.A. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32:1–14. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

