HepaCAM controls astrocyte self-organization and coupling
- PMID: 34171291
- PMCID: PMC8547372
- DOI: 10.1016/j.neuron.2021.05.025
HepaCAM controls astrocyte self-organization and coupling
Abstract
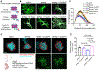
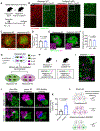
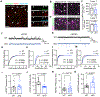
Astrocytes extensively infiltrate the neuropil to regulate critical aspects of synaptic development and function. This process is regulated by transcellular interactions between astrocytes and neurons via cell adhesion molecules. How astrocytes coordinate developmental processes among one another to parse out the synaptic neuropil and form non-overlapping territories is unknown. Here we identify a molecular mechanism regulating astrocyte-astrocyte interactions during development to coordinate astrocyte morphogenesis and gap junction coupling. We show that hepaCAM, a disease-linked, astrocyte-enriched cell adhesion molecule, regulates astrocyte competition for territory and morphological complexity in the developing mouse cortex. Furthermore, conditional deletion of Hepacam from developing astrocytes significantly impairs gap junction coupling between astrocytes and disrupts the balance between synaptic excitation and inhibition. Mutations in HEPACAM cause megalencephalic leukoencephalopathy with subcortical cysts in humans. Therefore, our findings suggest that disruption of astrocyte self-organization mechanisms could be an underlying cause of neural pathology.
Keywords: astrocyte; astrocyte development; astrocyte-synapse interaction; cell adhesion; connexin; gap junction; synapse; tiling.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





Comment in
-
HepaCAM shapes astrocyte territories, stabilizes gap-junction coupling, and influences neuronal excitability.Neuron. 2021 Aug 4;109(15):2365-2367. doi: 10.1016/j.neuron.2021.07.010. Neuron. 2021. PMID: 34352210
References
-
- Arshadi C, Eddison M, Günther U, Harrington KIS, and Ferreira TA (2020). SNT: A Unifying Toolbox for Quantification of Neuronal Anatomy. bioRxiv, 2020.2007.2013.179325 - PubMed
-
- Beahm DL, Oshima A, Gaietta GM, Hand GM, Smock AE, Zucker SN, Toloue MM, Chandrasekhar A, Nicholson BJ, and Sosinsky GE (2006). Mutation of a conserved threonine in the third transmembrane helix of alpha- and beta-connexins creates a dominant-negative closed gap junction channel. J Biol Chem 281, 7994–8009. - PubMed
-
- Beardslee MA, Laing JG, Beyer EC, and Saffitz JE (1998). Rapid turnover of connexin43 in the adult rat heart. Circ Res 83, 629–635. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

