Using focal cooling to link neural dynamics and behavior
- PMID: 34171292
- PMCID: PMC8376768
- DOI: 10.1016/j.neuron.2021.05.029
Using focal cooling to link neural dynamics and behavior
Abstract
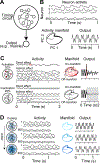
Establishing a causal link between neural function and behavioral output has remained a challenging problem. Commonly used perturbation techniques enable unprecedented control over intrinsic activity patterns and can effectively identify crucial circuit elements important for specific behaviors. However, these approaches may severely disrupt activity, precluding an investigation into the behavioral relevance of moment-to-moment neural dynamics within a specified brain region. Here we discuss the application of mild focal cooling to slow down intrinsic neural circuit activity while preserving its overall structure. Using network modeling and examples from multiple species, we highlight the power and versatility of focal cooling for understanding how neural dynamics control behavior and argue for its wider adoption within the systems neuroscience community.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





Similar articles
-
Navigating the Neural Space in Search of the Neural Code.Neuron. 2017 Mar 8;93(5):1003-1014. doi: 10.1016/j.neuron.2017.02.019. Neuron. 2017. PMID: 28279349 Review.
-
The Circuit Motif as a Conceptual Tool for Multilevel Neuroscience.Trends Neurosci. 2018 Mar;41(3):128-136. doi: 10.1016/j.tins.2018.01.002. Trends Neurosci. 2018. PMID: 29397990 Review.
-
From regions to connections and networks: new bridges between brain and behavior.Curr Opin Neurobiol. 2016 Oct;40:1-7. doi: 10.1016/j.conb.2016.05.003. Epub 2016 May 19. Curr Opin Neurobiol. 2016. PMID: 27209150 Free PMC article. Review.
-
New approaches to neural circuits in behavior.Learn Mem. 2012 Aug 16;19(9):385-90. doi: 10.1101/lm.025049.111. Learn Mem. 2012. PMID: 22904369 Review.
-
More Than a Small Brain: The Importance of Studying Neural Function during Development.J Neurosci. 2024 Nov 27;44(48):e1367242024. doi: 10.1523/JNEUROSCI.1367-24.2024. J Neurosci. 2024. PMID: 39603806 Free PMC article. Review.
Cited by
-
Focal Cooling for Drug-Resistant Epilepsy: A Review.JAMA Neurol. 2022 Sep 1;79(9):937-944. doi: 10.1001/jamaneurol.2022.1936. JAMA Neurol. 2022. PMID: 35877102 Free PMC article. Review.
-
Sensorimotor delays constrain robust locomotion in a 3D kinematic model of fly walking.Elife. 2025 May 15;13:RP99005. doi: 10.7554/eLife.99005. Elife. 2025. PMID: 40372779 Free PMC article.
-
Excitatory and inhibitory neural dynamics jointly tune motion detection.Curr Biol. 2022 Sep 12;32(17):3659-3675.e8. doi: 10.1016/j.cub.2022.06.075. Epub 2022 Jul 21. Curr Biol. 2022. PMID: 35868321 Free PMC article.
-
Brain-computer interfaces as a causal probe for scientific inquiry.Trends Cogn Sci. 2025 Jul 28:S1364-6613(25)00180-9. doi: 10.1016/j.tics.2025.06.017. Online ahead of print. Trends Cogn Sci. 2025. PMID: 40731219 Review.
-
Model-based correction of rapid thermal confounds in fluorescence neuroimaging of targeted perturbation.Neurophotonics. 2024 Jan;11(1):014413. doi: 10.1117/1.NPh.11.1.014413. Epub 2024 Feb 16. Neurophotonics. 2024. PMID: 38371339 Free PMC article.
References
-
- Aubert B, Barate R, Boutigny D, Gaillard JM, Hicheur A, Karyotakis Y, Lees JP, Robbe P, Tisserand V, Zghiche A, et al. (2004). Limits on the decay-rate difference of neutral B mesons and on CP, T, and CPT violation in B(0–0)B oscillations. Phys Rev Lett 92, 181801. - PubMed
-
- Bakken HE, Kawasaki H, Oya H, Greenlee JD, and Howard MA 3rd (2003). A device for cooling localized regions of human cerebral cortex. Technical note. J Neurosurg 99, 604–608. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

