HIV-1 Tat and morphine decrease murine inter-male social interactions and associated oxytocin levels in the prefrontal cortex, amygdala, and hypothalamic paraventricular nucleus
- PMID: 34171549
- PMCID: PMC8277758
- DOI: 10.1016/j.yhbeh.2021.105008
HIV-1 Tat and morphine decrease murine inter-male social interactions and associated oxytocin levels in the prefrontal cortex, amygdala, and hypothalamic paraventricular nucleus
Abstract
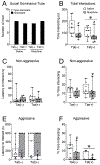
Many persons infected with HIV-1 (PWH) and opioid-dependent individuals experience deficits in sociability that interfere with daily living. Sociability is regulated by the prefrontal cortico-hippocampal-amygdalar circuit. Within this circuit HIV-1 trans-activator of transcription (HIV-1 Tat) and opioids can increase dendritic pathology and alter neuronal firing. Changes in sociability are also associated with dysregulation of hypothalamic neuropeptides such as oxytocin or corticotropin releasing factor (CRF) in the prefrontal cortico-hippocampal-amygdalar circuit. Accordingly, we hypothesized that the interaction of HIV-1 Tat and morphine would impair inter-male social interactions and disrupt oxytocin and CRF within the PFC and associated circuitry. Male mice were exposed to HIV-1 Tat for 8 weeks and administered saline or escalating doses of morphine twice daily (s.c.) during the last 2 weeks of HIV-1 Tat exposure. Tat attenuated aggressive interactions with an unknown intruder, whereas morphine decreased both non-aggressive and aggressive social interactions in the resident-intruder test. However, there was no effect of Tat or morphine on non-reciprocal interactions in the social interaction and novelty tests. Tat, but not morphine, decreased oxytocin levels in the PFC and amygdala, whereas both Tat and morphine decreased the percentage of oxytocin-immunoreactive neurons in the hypothalamic paraventricular nucleus (PVN). In Tat(+) or morphine-exposed mice, regional levels of CRF and oxytocin correlated with alterations in behavior in the social interaction and novelty tests. Overall, decreased expression of oxytocin in the prefrontal cortico-hippocampal-amygdalar circuit is associated with morphine- and HIV-Tat-induced deficits in social behavior.
Keywords: Aggression; Corticotropin releasing factor; Endogenous opioid system; HIV-associated neurocognitive disorders; Hippocampus; Opiate abuse; Paraventricular nucleus of the hypothalamus; Resident-intruder; Social anxiety; Social interaction.
Copyright © 2021 Elsevier Inc. All rights reserved.
Figures


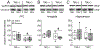




Similar articles
-
Depressive-like Behavior Is Accompanied by Prefrontal Cortical Innate Immune Fatigue and Dendritic Spine Losses after HIV-1 Tat and Morphine Exposure.Viruses. 2023 Feb 21;15(3):590. doi: 10.3390/v15030590. Viruses. 2023. PMID: 36992299 Free PMC article.
-
Central HIV-1 Tat exposure elevates anxiety and fear conditioned responses of male mice concurrent with altered mu-opioid receptor-mediated G-protein activation and β-arrestin 2 activity in the forebrain.Neurobiol Dis. 2016 Aug;92(Pt B):124-36. doi: 10.1016/j.nbd.2016.01.014. Epub 2016 Feb 1. Neurobiol Dis. 2016. PMID: 26845176 Free PMC article.
-
Disruption of the CRF1 receptor eliminates morphine-induced sociability deficits and firing of oxytocinergic neurons in male mice.Elife. 2025 Feb 5;13:RP100849. doi: 10.7554/eLife.100849. Elife. 2025. PMID: 39907358 Free PMC article.
-
Central vasopressin and oxytocin release: regulation of complex social behaviours.Prog Brain Res. 2008;170:261-76. doi: 10.1016/S0079-6123(08)00422-6. Prog Brain Res. 2008. PMID: 18655888 Review.
-
Interactions between the κ opioid system, corticotropin-releasing hormone and oxytocin in partner loss.Philos Trans R Soc Lond B Biol Sci. 2022 Aug 29;377(1858):20210061. doi: 10.1098/rstb.2021.0061. Epub 2022 Jul 11. Philos Trans R Soc Lond B Biol Sci. 2022. PMID: 35858099 Free PMC article. Review.
Cited by
-
HIV-1 Tat reduces apical dendritic spine density throughout the trisynaptic pathway in the hippocampus of male transgenic mice.Neurosci Lett. 2022 Jun 21;782:136688. doi: 10.1016/j.neulet.2022.136688. Epub 2022 May 17. Neurosci Lett. 2022. PMID: 35595189 Free PMC article.
-
HIV-1 Tat protein alters medial prefrontal cortex neuronal activity and recognition memory.iScience. 2025 Feb 22;28(3):112075. doi: 10.1016/j.isci.2025.112075. eCollection 2025 Mar 21. iScience. 2025. PMID: 40160418 Free PMC article.
-
Neurodegeneration Within the Amygdala Is Differentially Induced by Opioid and HIV-1 Tat Exposure.Front Neurosci. 2022 May 4;16:804774. doi: 10.3389/fnins.2022.804774. eCollection 2022. Front Neurosci. 2022. PMID: 35600626 Free PMC article.
-
Functional modular networks identify the pivotal genes associated with morphine addiction and potential drug therapies.BMC Anesthesiol. 2023 May 3;23(1):151. doi: 10.1186/s12871-023-02111-2. BMC Anesthesiol. 2023. Retraction in: BMC Anesthesiol. 2024 Mar 9;24(1):97. doi: 10.1186/s12871-024-02482-0. PMID: 37138216 Free PMC article. Retracted.
-
A new perspective on HIV: effects of HIV on brain-heart axis.Front Cardiovasc Med. 2023 Aug 4;10:1226782. doi: 10.3389/fcvm.2023.1226782. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 37600062 Free PMC article. Review.
References
-
- Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE, 2005. Does drug abuse alter microglial phenotype and cell turnover in the context of advancing HIV infection? Neuropathol.Appl.Neurobiol. 31, 325–338. - PubMed
-
- Atweh SF, Kuhar MJ, 1983. Distribution and physiological significance of opioid receptors in the brain. Br Med Bull 39, 47–52. - PubMed
-
- Avitsur R, Stark JL, Sheridan JF, 2001. Social stress induces glucocorticoid resistance in subordinate animals. Horm Behav 39, 247–257. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

