Strand discrimination in DNA mismatch repair
- PMID: 34171627
- PMCID: PMC8785607
- DOI: 10.1016/j.dnarep.2021.103161
Strand discrimination in DNA mismatch repair
Abstract
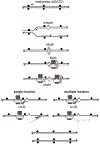
DNA mismatch repair (MMR) corrects non-Watson-Crick basepairs generated by replication errors, recombination intermediates, and some forms of chemical damage to DNA. In MutS and MutL homolog-dependent MMR, damaged bases do not identify the error-containing daughter strand that must be excised and resynthesized. In organisms like Escherichia coli that use methyl-directed MMR, transient undermethylation identifies the daughter strand. For other organisms, growing in vitro and in vivo evidence suggest that strand discrimination is mediated by DNA replication-associated daughter strand nicks that direct asymmetric loading of the replicative clamp (the β-clamp in bacteria and the proliferating cell nuclear antigen, PCNA, in eukaryotes). Structural modeling suggests that replicative clamps mediate strand specificity either through the ability of MutL homologs to recognize the fixed orientation of the daughter strand relative to one face of the replicative clamps or through parental strand-specific diffusion of replicative clamps on DNA, which places the daughter strand in the MutL homolog endonuclease active site. Finally, identification of bacteria that appear to lack strand discrimination mediated by a replicative clamp and a pre-existing nick suggest that other strand discrimination mechanisms exist or that these organisms perform MMR by generating a double-stranded DNA break intermediate, which may be analogous to NucS-mediated MMR.
Keywords: DNA mismatch repair; DNA mispair; Strand discrimination.
Copyright © 2021 The Author. Published by Elsevier B.V. All rights reserved.
Figures
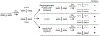
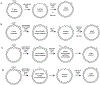
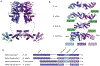

Similar articles
-
Influence of oxidized purine processing on strand directionality of mismatch repair.J Biol Chem. 2015 Apr 17;290(16):9986-99. doi: 10.1074/jbc.M114.629907. Epub 2015 Feb 18. J Biol Chem. 2015. PMID: 25694431 Free PMC article.
-
MutL traps MutS at a DNA mismatch.Proc Natl Acad Sci U S A. 2015 Sep 1;112(35):10914-9. doi: 10.1073/pnas.1505655112. Epub 2015 Aug 17. Proc Natl Acad Sci U S A. 2015. PMID: 26283381 Free PMC article.
-
Ligation of newly replicated DNA controls the timing of DNA mismatch repair.Curr Biol. 2021 Mar 22;31(6):1268-1276.e6. doi: 10.1016/j.cub.2020.12.018. Epub 2021 Jan 7. Curr Biol. 2021. PMID: 33417883 Free PMC article.
-
Evolution of the methyl directed mismatch repair system in Escherichia coli.DNA Repair (Amst). 2016 Feb;38:32-41. doi: 10.1016/j.dnarep.2015.11.016. Epub 2015 Dec 2. DNA Repair (Amst). 2016. PMID: 26698649 Free PMC article. Review.
-
DNA mismatch repair and mutation avoidance pathways.J Cell Physiol. 2002 Apr;191(1):28-41. doi: 10.1002/jcp.10077. J Cell Physiol. 2002. PMID: 11920679 Review.
Cited by
-
Strand asymmetry influences mismatch resolution during a single-strand annealing.Genome Biol. 2022 Apr 12;23(1):93. doi: 10.1186/s13059-022-02665-3. Genome Biol. 2022. PMID: 35414014 Free PMC article.
-
G-Quadruplex Formed by the Promoter Region of the hTERT Gene: Structure-Driven Effects on DNA Mismatch Repair Functions.Biomedicines. 2022 Aug 3;10(8):1871. doi: 10.3390/biomedicines10081871. Biomedicines. 2022. PMID: 36009419 Free PMC article.
-
Tripterhyponoid A from Tripterygium hypoglaucum Inhibiting MRSA by Multiple Mechanisms.Molecules. 2025 Jun 10;30(12):2539. doi: 10.3390/molecules30122539. Molecules. 2025. PMID: 40572502 Free PMC article.
-
Action-At-A-Distance in DNA Mismatch Repair: Mechanistic Insights and Models for How DNA and Repair Proteins Facilitate Long-Range Communication.Biomolecules. 2024 Nov 13;14(11):1442. doi: 10.3390/biom14111442. Biomolecules. 2024. PMID: 39595618 Free PMC article. Review.
-
A complete DNA repair system assembled by two endosymbionts restores heat tolerance of the insect host.Proc Natl Acad Sci U S A. 2024 Dec 17;121(51):e2415651121. doi: 10.1073/pnas.2415651121. Epub 2024 Dec 10. Proc Natl Acad Sci U S A. 2024. PMID: 39656210 Free PMC article.
References
-
- Iyer RR, Pluciennik A, Burdett V, Modrich PL, DNA mismatch repair: functions and mechanisms. Chem Rev 106, 302–323 (2006). - PubMed
-
- Marinus MG, Morris NR, Pleiotropic effects of a DNA adenine methylation mutation (dam-3) in Escherichia coli K12. Mutat Res 28, 15–26 (1975). - PubMed
-
- Marinus MG, Morris NR, Biological function for 6-methyladenine residues in the DNA of Escherichia coli K12. J Mol Biol 85, 309–322 (1974). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

