Longitudinal study on antibiotic susceptibility in commensal E. coli from geese raised in free-range production systems
- PMID: 34171653
- PMCID: PMC8243015
- DOI: 10.1016/j.psj.2021.101230
Longitudinal study on antibiotic susceptibility in commensal E. coli from geese raised in free-range production systems
Abstract
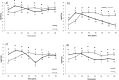
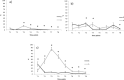
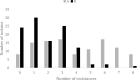
The transmission of antimicrobial resistance bacteria from animals to humans has become an important concern. The extended-spectrum beta-lactamase (ESBL) -AmpC- producing Escherichia coli (ESBL-AmpC EC) and quinolones resistant E. coli are of particular interest. The present study aimed to evaluate the load and prevalence of antibiotic-resistant commensal E. coli along the goose production cycle on 2 free-range farms in central Italy. On A farm, oxytetracycline was administered, while the B farm did not use antibiotics during the geese productive cycle. One hundred geese of 1-day-old from the same batch were divided into the two farms. At hatching, the animals showed an average of E. coli loads was 6.83 ± 0.48 log CFU/g, and 0.28 ± 0.28, 0, 5.12 ± 0.54 log CFU/g for E. coli resistant to nalidixic acid (E. colinal), to cefotaxime (E. colicef) and to tetracyclines (E. colitet), respectively. The loads of E. coli, E. colinal, E. colicef and E. colitet on 224 environmental faecal pools were determined at 8 time points. Antimicrobial susceptibility and molecular characterization of E. colicef isolates were performed. The ANOVA was used to assess the difference in bacterial loads between the two farms. We described more than 50% of resistances for tetracyclines in both farms, and sulphonamides and cephazolin in the A farm. The loads of E. coli and E. colinal in faeces were estimated at approximately 6-7 log (CFU/g) and 5-6 log (CFU/g) in the two farms, respectively. The average load of extended-spectrum beta-lactamase Escherichia coli (ESBL EC) in goose faeces varied broadly along the production cycle: in the first weeks, a sharp increase was observed in both farms, while later on A farm, the burden of ESBL EC remained steady until the end of the production cycle and on B farm the load dramatically decreased from 6 wk of age onward. An increase in the proportion of E. colinal was observed on A farm shortly after the antibiotic administration. Our study shows that the dynamics of antibiotic-resistant E. coli in farmed geese are similar to the ones observed in broilers. However, the risk of the emergence of antibiotic-resistant commensal E. coli, might be mitigated by the adoption of good management practices, including prudent use of antibiotics.
Keywords: antibiotic susceptibility; commensal E. coli; free-range farm; goose.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Bertelloni F., Salvadori C., Moni A., Cerri D., Mani P., Ebani V.V. Antimicrobial resistance in Enterococcus spp. isolated from laying hens of backyard poultry flocks. Ann. Agric. Environ. Med. 2015;22:4. - PubMed
-
- Bortolaia V., Bisgaard M., Bojesen A.M. Distribution and possible transmission of ampicillin- and nalidixic acid-resistant Escherichia coli within the broiler industry. Vet. Microbiol. 2010;142:379–386. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

