Point-of-care CRISPR-Cas-assisted SARS-CoV-2 detection in an automated and portable droplet magnetofluidic device
- PMID: 34171821
- PMCID: PMC8170879
- DOI: 10.1016/j.bios.2021.113390
Point-of-care CRISPR-Cas-assisted SARS-CoV-2 detection in an automated and portable droplet magnetofluidic device
Abstract
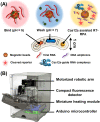
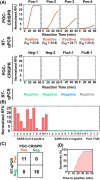
In the fight against COVID-19, there remains an unmet need for point-of-care (POC) diagnostic testing tools that can rapidly and sensitively detect the causative SARS-CoV-2 virus to control disease transmission and improve patient management. Emerging CRISPR-Cas-assisted SARS-CoV-2 detection assays are viewed as transformative solutions for POC diagnostic testing, but their lack of streamlined sample preparation and full integration within an automated and portable device hamper their potential for POC use. We report herein POC-CRISPR - a single-step CRISPR-Cas-assisted assay that incoporates sample preparation with minimal manual operation via facile magnetic-based nucleic acid concentration and transport. Moreover, POC-CRISPR has been adapted into a compact thermoplastic cartridge within a palm-sized yet fully-integrated and automated device. During analytical evaluation, POC-CRISPR was able detect 1 genome equivalent/μL SARS-CoV-2 RNA from a sample volume of 100 μL in < 30 min. When evaluated with 27 unprocessed clinical nasopharyngeal swab eluates that were pre-typed by standard RT-qPCR (Cq values ranged from 18.3 to 30.2 for the positive samples), POC-CRISPR achieved 27 out of 27 concordance and could detect positive samples with high SARS-CoV-2 loads (Cq < 25) in 20 min.
Keywords: CRISPR; Point-of-Care; Sensors; Viruses.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
A one-step, one-pot CRISPR nucleic acid detection platform (CRISPR-top): Application for the diagnosis of COVID-19.Talanta. 2021 Oct 1;233:122591. doi: 10.1016/j.talanta.2021.122591. Epub 2021 Jun 12. Talanta. 2021. PMID: 34215080 Free PMC article.
-
Contamination-free visual detection of SARS-CoV-2 with CRISPR/Cas12a: A promising method in the point-of-care detection.Biosens Bioelectron. 2020 Dec 1;169:112642. doi: 10.1016/j.bios.2020.112642. Epub 2020 Sep 20. Biosens Bioelectron. 2020. PMID: 32979593 Free PMC article.
-
Short-Time Preamplification-Assisted One-Pot CRISPR Nucleic Acid Detection Method with Portable Self-Heating Equipment for Point-of-Care Diagnosis.Anal Chem. 2025 Jan 14;97(1):658-666. doi: 10.1021/acs.analchem.4c05026. Epub 2025 Jan 4. Anal Chem. 2025. PMID: 39754554
-
CRISPR is a useful biological tool for detecting nucleic acid of SARS-CoV-2 in human clinical samples.Biomed Pharmacother. 2021 Aug;140:111772. doi: 10.1016/j.biopha.2021.111772. Epub 2021 May 27. Biomed Pharmacother. 2021. PMID: 34062417 Free PMC article. Review.
-
The nucleic acid detection using CRISPR/Cas biosensing system with micro-nano modality for point-of-care applications.Talanta. 2025 May 1;286:127457. doi: 10.1016/j.talanta.2024.127457. Epub 2024 Dec 24. Talanta. 2025. PMID: 39724853 Review.
Cited by
-
Needs, Challenges and Countermeasures of SARS-CoV-2 Surveillance in Cold-Chain Foods and Packaging to Prevent Possible COVID-19 Resurgence: A Perspective from Advanced Detections.Viruses. 2022 Dec 30;15(1):120. doi: 10.3390/v15010120. Viruses. 2022. PMID: 36680157 Free PMC article. Review.
-
Recent advances in the biosensors application for the detection of bacteria and viruses in wastewater.J Environ Chem Eng. 2022 Feb;10(1):107070. doi: 10.1016/j.jece.2021.107070. Epub 2021 Dec 24. J Environ Chem Eng. 2022. PMID: 34976725 Free PMC article. Review.
-
Mechanisms, Techniques and Devices of Airborne Virus Detection: A Review.Int J Environ Res Public Health. 2023 Apr 11;20(8):5471. doi: 10.3390/ijerph20085471. Int J Environ Res Public Health. 2023. PMID: 37107752 Free PMC article. Review.
-
Development of quantitative wastewater surveillance models facilitated the precise epidemic management of COVID-19.Sci Total Environ. 2023 Jan 20;857(Pt 1):159357. doi: 10.1016/j.scitotenv.2022.159357. Epub 2022 Oct 12. Sci Total Environ. 2023. PMID: 36240917 Free PMC article.
-
Accuracy of clustered regularly interspaced short palindromic repeats (CRISPR) to diagnose COVID-19, a meta-analysis.Microb Pathog. 2022 Apr;165:105498. doi: 10.1016/j.micpath.2022.105498. Epub 2022 Mar 25. Microb Pathog. 2022. PMID: 35341958 Free PMC article.
References
-
- Arizti-Sanz J., Freije C.A., Stanton A.C., Petros B.A., Boehm C.K., Siddiqui S., Shaw B.M., Adams G., Kosoko-Thoroddsen T.-S.F., Kemball M.E., Uwanibe J.N., Ajogbasile F.V., Eromon P.E., Gross R., Wronka L., Caviness K., Hensley L.E., Bergman N.H., MacInnis B.L., Happi C.T., Lemieux J.E., Sabeti P.C., Myhrvold C. Streamlined inactivation, amplification, and Cas13-based detection of SARS-CoV-2. Nat. Commun. 2020;11:5921. doi: 10.1038/s41467-020-19097-x. - DOI - PMC - PubMed
-
- Basu A., Zinger T., Inglima K., Woo K., Atie O., Yurasits L., See B., Aguero-Rosenfeld M.E. Performance of Abbott ID now COVID-19 rapid nucleic acid amplification test using nasopharyngeal swabs transported in viral transport media and dry nasal swabs in a New York city academic institution. J. Clin. Microbiol. 2020;58 doi: 10.1128/JCM.01136-20. - DOI - PMC - PubMed
-
- Brendish N.J., Poole S., Naidu V.V., Mansbridge C.T., Norton N.J., Wheeler H., Presland L., Kidd S., Cortes N.J., Borca F., Phan H., Babbage G., Visseaux B., Ewings S., Clark T.W. Clinical impact of molecular point-of-care testing for suspected COVID-19 in hospital (COV-19POC): a prospective, interventional, non-randomised, controlled study. Lancet Respir. Med. 2020;8:1192–1200. doi: 10.1016/S2213-2600(20)30454-9. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

