Randomised, controlled trial of erenumab for the prevention of episodic migraine in patients from Asia, the Middle East, and Latin America: The EMPOwER study
- PMID: 34171973
- PMCID: PMC8592099
- DOI: 10.1177/03331024211024160
Randomised, controlled trial of erenumab for the prevention of episodic migraine in patients from Asia, the Middle East, and Latin America: The EMPOwER study
Abstract
Objective: EMPOwER, a double-blind, randomised, phase 3 study, evaluated the efficacy and safety of erenumab in adults with episodic migraine from Asia, the Middle East, and Latin America.
Methods: Randomised patients (N = 900) received monthly subcutaneous injections of placebo, erenumab 70 mg, or 140 mg (3:3:2) for 3 months. Primary endpoint was change from baseline in monthly migraine days at Month 3. Other endpoints included achievement of ≥50%, ≥75%, and 100% reduction in monthly migraine days, change in monthly acute migraine-specific medication treatment days, patient-reported outcomes, and safety assessment.
Results: At baseline, mean (standard deviation) age was 37.5 (9.9) years, 81.9% were women, and monthly migraine days was 8.2 (2.8). At Month 3, change from baseline in monthly migraine days (primary endpoint) was -3.1, -4.2, and -4.8 days for placebo, erenumab 70 mg, and erenumab 140 mg, respectively, with a statistically significant difference for erenumab versus placebo (P = 0.002 [70 mg], P < 0.001 [140 mg]). Both erenumab doses were also significantly superior to placebo on all secondary endpoints, including the proportion of patients achieving ≥50% reduction from baseline in monthly migraine days, change from baseline in monthly acute migraine-specific medication treatment days and change from baseline in the Headache Impact Test-6™ scores. The safety profile of erenumab was comparable with placebo; no new safety signals were observed.
Conclusions: This study of erenumab in patients with episodic migraine from Asia, the Middle East, and Latin America met all primary and secondary endpoints. A consistent numerical benefit was observed with erenumab 140 mg versus erenumab 70 mg across all efficacy endpoints. These findings extend evidence of erenumab's efficacy and safety to patients under-represented in previous trials.ClinicalTrials.gov identifier: NCT03333109.
Keywords: Asia; Latin America; calcitonin gene-related peptide; episodic migraine; erenumab; randomised controlled trial.
Conflict of interest statement
Figures

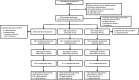

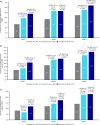
Similar articles
-
Efficacy and Tolerability of Erenumab for Prevention of Episodic Migraine in India.Ann Indian Acad Neurol. 2022 May-Jun;25(3):433-440. doi: 10.4103/aian.aian_199_22. Epub 2022 Jun 9. Ann Indian Acad Neurol. 2022. PMID: 35936611 Free PMC article.
-
A Randomized Phase 2 Study of Erenumab for the Prevention of Episodic Migraine in Japanese Adults.Headache. 2019 Nov;59(10):1731-1742. doi: 10.1111/head.13652. Epub 2019 Oct 14. Headache. 2019. PMID: 31612482 Free PMC article. Clinical Trial.
-
A phase 3, randomised, placebo-controlled study of erenumab for the prevention of chronic migraine in patients from Asia: the DRAGON study.J Headache Pain. 2022 Nov 21;23(1):146. doi: 10.1186/s10194-022-01514-9. J Headache Pain. 2022. PMID: 36404301 Free PMC article. Clinical Trial.
-
Unveiling the efficacy and safety of Erenumab, a monoclonal antibody targeting calcitonin gene-related peptide (CGRP) receptor, in patients with chronic and episodic migraine: a GRADE-assessed systematic review and meta-analysis of randomized clinical trials with subgroup analysis.Head Face Med. 2025 Mar 26;21(1):19. doi: 10.1186/s13005-025-00494-w. Head Face Med. 2025. PMID: 40134001 Free PMC article.
-
Erenumab for episodic migraine.Pain Manag. 2022 Jul;12(5):587-594. doi: 10.2217/pmt-2021-0077. Epub 2022 Mar 22. Pain Manag. 2022. PMID: 35313740 Review.
Cited by
-
Efficacy and Tolerability of Erenumab for Prevention of Episodic Migraine in India.Ann Indian Acad Neurol. 2022 May-Jun;25(3):433-440. doi: 10.4103/aian.aian_199_22. Epub 2022 Jun 9. Ann Indian Acad Neurol. 2022. PMID: 35936611 Free PMC article.
-
CGRP-Targeted Therapy for Episodic and Chronic Cluster Headache.Curr Pain Headache Rep. 2022 Sep;26(9):667-675. doi: 10.1007/s11916-022-01070-6. Epub 2022 Jul 26. Curr Pain Headache Rep. 2022. PMID: 35881279 Review.
-
Repurposed versus disease-specific medicinals for the prophylaxis of migraine: an updated systematic review.Pain Manag. 2025 Jul;15(7):425-439. doi: 10.1080/17581869.2025.2509474. Epub 2025 May 30. Pain Manag. 2025. PMID: 40447296
-
Calcitonin Gene-Related Peptide (CGRP)-Targeted Monoclonal Antibodies and Antagonists in Migraine: Current Evidence and Rationale.BioDrugs. 2022 May;36(3):341-358. doi: 10.1007/s40259-022-00530-0. Epub 2022 Apr 27. BioDrugs. 2022. PMID: 35476215 Free PMC article. Review.
-
Increased infection risk in patients on preventive CGRP-targeting therapies- a meta-analysis and clinical effect assessment.J Headache Pain. 2025 Apr 25;26(1):88. doi: 10.1186/s10194-025-02040-0. J Headache Pain. 2025. PMID: 40281424 Free PMC article.
References
-
- Woldeamanuel YW, Cowan RP. Migraine affects 1 in 10 people worldwide featuring recent rise: a systematic review and meta-analysis of community-based studies involving 6 million participants. J Neur Sc 2017; 372: 307–315. - PubMed
-
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017; 390: 1211–1259. - PMC - PubMed
-
- Buse DC, Scher AI, Dodick DW, et al.. Impact of migraine on the family: perspectives of people with migraine and their spouse/domestic partner in the CaMEO study. Mayo Clinic Proceedings 2016; 91: 596–611. - PubMed
-
- Blumenfeld AM, Varon SF, Wilcox TK, et al.. Disability, HRQoL and resource use among chronic and episodic migraineurs: results from the International Burden of Migraine Study (IBMS). Cephalalgia 2011; 31: 301–315. - PubMed
-
- Bonafede M, Sapra S, Shah N, et al.. Direct and indirect healthcare resource utilization and costs among migraine patients in the United States. Headache 2018; 58: 700–714. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

