A novel case of concurrent occurrence of demyelinating-polyneuropathy-causing PMP22 duplication and SOX10 gene mutation producing severe hypertrophic neuropathy
- PMID: 34171997
- PMCID: PMC8228911
- DOI: 10.1186/s12883-021-02256-y
A novel case of concurrent occurrence of demyelinating-polyneuropathy-causing PMP22 duplication and SOX10 gene mutation producing severe hypertrophic neuropathy
Abstract
Background: Hereditary motor and sensory neuropathy, also referred to as Charcot-Marie-Tooth disease (CMT), is most often caused by a duplication of the peripheral myelin protein 22 (PMP22) gene. This duplication causes CMT type 1A (CMT1A). CMT1A rarely occurs in combination with other hereditary neuromuscular disorders. However, such rare genetic coincidences produce a severe phenotype and have been reported in terms of "double trouble" overlapping syndrome. Waardenburg syndrome (WS) is the most common form of a hereditary syndromic deafness. It is primarily characterized by pigmentation anomalies and classified into four major phenotypes. A mutation in the SRY sex determining region Y-box 10 (SOX10) gene causes WS type 2 or 4 and peripheral demyelinating neuropathy, central dysmyelinating leukodystrophy, WS, and Hirschsprung disease. We describe a 11-year-old boy with extreme hypertrophic neuropathy because of a combination of CMT1A and WS type 2. This is the first published case on the co-occurrence of CMT1A and WS type 2.
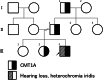
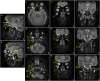
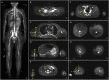
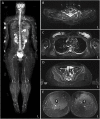
Case presentation: The 11-year-old boy presented with motor developmental delay and a deterioration in unstable walking at 6 years of age. In addition, he had congenital hearing loss and heterochromia iridis. The neurological examination revealed weakness in the distal limbs with pes cavus. He was diagnosed with CMT1A by the fluorescence in situ hybridization method. His paternal pedigree had a history of CMT1A. However, no family member had congenital hearing loss. His clinical manifestation was apparently severe than those of his relatives with CMT1A. In addition, a whole-body magnetic resonance neurography revealed an extreme enlargement of his systemic cranial and spinal nerves. Subsequently, a genetic analysis revealed a heterozygous frameshift mutation c.876delT (p.F292Lfs*19) in the SOX10 gene. He was eventually diagnosed with WS type 2.
Conclusions: We described a patient with a genetically confirmed overlapping diagnoses of CMT1A and WS type 2. The double trouble with the genes created a significant impact on the peripheral nerves system. Severe phenotype in the proband can be attributed to the cumulative effect of mutations in both PMP22 and SOX10 genes, responsible for demyelinating neuropathy.
Keywords: Charcot–Marie–tooth disease; Hypertrophic neuropathy; MR neurography; PMP22; SOX10; Waardenburg syndrome; Whole-body MRI.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

