The impact of tube replacement timing during LCIG therapy on PEG-J associated adverse events: a retrospective multicenter observational study
- PMID: 34172002
- PMCID: PMC8228941
- DOI: 10.1186/s12883-021-02269-7
The impact of tube replacement timing during LCIG therapy on PEG-J associated adverse events: a retrospective multicenter observational study
Abstract
Background: Levodopa-carbidopa intestinal gel (LCIG) treatment, a unique drug delivery system for patients with advanced Parkinson's disease (PD), is covered by health insurance in Japan since September 2016. Various LCIG procedure/device-associated adverse events (AEs) have been reported; however, reports on their treatment have been limited. This is the first multicenter study to clarify the frequency and timing of device-related AEs.
Methods: Between September 2016 and December 2018, 104 patients introduced to the LCIG treatment for advanced PD in 11 hospitals were included. The patients' characteristics, AEs incidence, AEs time, and tube exchange time were investigated.
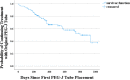
Results: The median follow-up period was 21.5 months. Minor AE cases were 29.4%, whereas major AE cases were 43.1%. Majority of major AEs (n = 55, 94.8%) were managed with endoscopic treatment, such as tube exchange. Few severe AEs required surgical treatment (n =3, 5.2%). The mean (range) exposure to percutaneous endoscopic gastrojejunostomy (PEG-J) was 14.7 (0-33) months. One year after the LCIG treatment introduction, 55 patients (54.0%) retained the original PEG-J tube. The mean PEG-J tube exchange time was 10.8 ± 7.0 months in all patients, 11.6 ± 4.7 and 10.5 ± 7.7 months in patients with scheduled exchange and who underwent exchange due to AEs, respectively.
Conclusions: Some device-related AEs occurred during the LCIG treatment; however, only few were serious, most of which could be treated with simple procedures or tube replacement with endoscopy. Therefore, the LCIG treatment is feasible and safe and is a unique treatment option for PD, requiring endoscopists' understanding and cooperation.
Keywords: Levodopa–carbidopa intestinal gel; Parkinson’s disease; Percutaneous endoscopic gastrojejunostomy.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures
Similar articles
-
Safety and effectiveness of levodopa-carbidopa intestinal gel for advanced Parkinson's disease: A large single-center study.Rev Neurol (Paris). 2020 May;176(4):268-276. doi: 10.1016/j.neurol.2019.07.024. Epub 2019 Oct 23. Rev Neurol (Paris). 2020. PMID: 31668287
-
Problems related to levodopa-carbidopa intestinal gel treatment in advanced Parkinson's disease.Brain Behav. 2017 Jun 5;7(7):e00737. doi: 10.1002/brb3.737. eCollection 2017 Jul. Brain Behav. 2017. PMID: 28729942 Free PMC article.
-
Adverse effects of levodopa/carbidopa intrajejunal gel treatment: A single-center long-term follow-up study.Acta Neurol Scand. 2022 Nov;146(5):537-544. doi: 10.1111/ane.13675. Epub 2022 Jul 28. Acta Neurol Scand. 2022. PMID: 35903042 Free PMC article.
-
Current Practices for Outpatient Initiation of Levodopa-Carbidopa Intestinal Gel for Management of Advanced Parkinson's Disease in the United States.Adv Ther. 2019 Sep;36(9):2233-2246. doi: 10.1007/s12325-019-01014-4. Epub 2019 Jul 5. Adv Ther. 2019. PMID: 31278691 Free PMC article. Review.
-
The Advantages of Levodopa-Carbidopa Intestinal Gel for Patients with Advanced Parkinson's Disease: A Systematic Review.Drug Des Devel Ther. 2020 Feb 26;14:845-854. doi: 10.2147/DDDT.S229621. eCollection 2020. Drug Des Devel Ther. 2020. PMID: 32161444 Free PMC article.
Cited by
-
Access to device-aided therapies in advanced Parkinson's disease: navigating clinician biases, patient preference, and prognostic uncertainty.J Neural Transm (Vienna). 2023 Nov;130(11):1411-1432. doi: 10.1007/s00702-023-02668-9. Epub 2023 Jul 12. J Neural Transm (Vienna). 2023. PMID: 37436446 Free PMC article. Review.
-
Why do 'OFF' periods still occur during continuous drug delivery in Parkinson's disease?Transl Neurodegener. 2022 Oct 13;11(1):43. doi: 10.1186/s40035-022-00317-x. Transl Neurodegener. 2022. PMID: 36229860 Free PMC article. Review.
-
Transgastric Jejunostomy (PEG-J) for Continuous Infusion of Levodopa-Carbidopa Intestinal Gel: An Approach for Parkinson's Disease Treatment.Med Sci Monit. 2023 Aug 12;29:e941285. doi: 10.12659/MSM.941285. Med Sci Monit. 2023. PMID: 37571821 Free PMC article.
-
A knotty problem: phytobezoar and small bowel occlusion as a complication of a gastro-jejunal catheter for continuous Duodopa infusion: a case report.J Surg Case Rep. 2022 Mar 26;2022(3):rjac118. doi: 10.1093/jscr/rjac118. eCollection 2022 Mar. J Surg Case Rep. 2022. PMID: 35355578 Free PMC article.
-
Nasojejunal Tube Placement for Levodopa-carbidopa Intestinal Gel Treatment by Neurologists in Patients with Advanced Parkinson's Disease: A Retrospective Observational Study.Intern Med. 2025 May 1;64(9):1315-1320. doi: 10.2169/internalmedicine.4394-24. Epub 2024 Sep 27. Intern Med. 2025. PMID: 39343563 Free PMC article.
References
-
- Obeso JA, Grandas F, Vaamonde J, Luquin MR, Artieda J, Lera G, et al. Motor complications associated with chronic levodopa therapy in Parkinson’s disease. Neurology. 1989;39:11–19. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical