Bioactive fractions and compound of Ardisia crispa roots exhibit anti-arthritic properties mediated via angiogenesis inhibition in vitro
- PMID: 34172047
- PMCID: PMC8235828
- DOI: 10.1186/s12906-021-03341-y
Bioactive fractions and compound of Ardisia crispa roots exhibit anti-arthritic properties mediated via angiogenesis inhibition in vitro
Abstract
Background: Ardisia crispa (Thunb.) A.DC (Primulaceae), is a medicinal herb traditionally used by Asian people as remedies to cure inflammatory related diseases, including rheumatism. The plant roots possess various pharmacological activities including antipyretic, anti-inflammation and antitumor. Previous phytochemical studies of the plant roots have identified long chain alkyl-1,4-benzoquinones as major constituents, together with other phytochemicals. Hexane fraction of the plant roots (ACRH), was previously reported with anti-angiogenic and anti-arthritic properties, while its effect on their anti-arthritic in vitro, is yet unrevealed. Considering the significance of angiogenesis inhibition in developing new anti-arthritic agent, thus we investigated the anti-arthritic potential of Ardisia crispa roots by suppressing angiogenesis, in vitro.
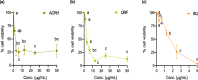
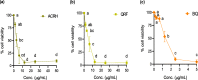
Methods: Ardisia crispa roots hexane extract (ACRH) was prepared from the plant roots using absolute n-hexane. ACRH was fractionated into quinone-rich fraction (QRF) and further isolated to yield benzoquinonoid compound (BQ), respectively. In vitro experiments using VEGF-induced human umbilical vein endothelial cells (HUVECs) and IL-1β-induced human fibroblast-like synoviocytes for rheumatoid arthritis (HFLS-RA) were performed to evaluate the effects of these samples on VEGF-induced HUVECs proliferation and tube formation, and towards IL-1β-induced HFLS-RA proliferation, invasion, and apoptosis, respectively. Therapeutic concentrations (0.05, 0.5, and 5 μg/mL) tested in this study were predetermined based on the IC50 values obtained from the MTT assay.
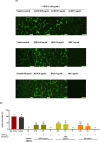
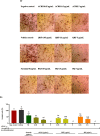
Results: ACRH, QRF, and BQ exerted concentration-independent antiproliferative effects on VEGF-induced HUVECs and IL-1β-induced HFLS-RA, with IC50 values at 1.09 ± 0.18, 3.85 ± 0.26, and 1.34 ± 0.16 μg/mL in HUVECs; and 3.60 ± 1.38, 4.47 ± 0.34, and 1.09 ± 0.09 μg/mL in HFLS-RA, respectively. Anti-angiogenic properties of these samples were verified via significant inhibition on VEGF-induced HUVECs tube formation, in a concentration-independent manner. The invasiveness of IL-1β-induced HFLS-RA was also significantly inhibited in a concentration-independent manner by all samples. ACRH and BQ, but not QRF, significantly enhanced the apoptosis of IL-1β-induced HFLS-RA elicited at their highest concentration (5 μg/mL) (P < 0.05).
Conclusions: These findings highlight the bioactive fractions and compound from Ardisia crispa roots as potential anti-arthritic agents by inhibiting both HUVECs and HFLS-RA's cellular functions in vitro, possibly mediated via their anti-angiogenic effects.
Keywords: Angiogenesis; Ardisia crispa; Human fibroblast-like synoviocytes for rheumatoid arthritis; Human umbilical vein endothelial cells; Rheumatoid arthritis.
Conflict of interest statement
The authors declare that they have no competing interest.
Figures
Similar articles
-
Ardisia crispa root hexane fraction suppressed angiogenesis in human umbilical vein endothelial cells (HUVECs) and in vivo zebrafish embryo model.Biomed Pharmacother. 2019 Oct;118:109221. doi: 10.1016/j.biopha.2019.109221. Epub 2019 Aug 26. Biomed Pharmacother. 2019. PMID: 31545225
-
Quinone-rich fraction of Ardisia crispa (Thunb.) A. DC roots alters angiogenic cascade in collagen-induced arthritis.Inflammopharmacology. 2021 Jun;29(3):771-788. doi: 10.1007/s10787-021-00816-9. Epub 2021 Jun 5. Inflammopharmacology. 2021. PMID: 34091811
-
The hexane fraction of Ardisia crispa Thunb. A. DC. roots inhibits inflammation-induced angiogenesis.BMC Complement Altern Med. 2013 Jan 8;13:5. doi: 10.1186/1472-6882-13-5. BMC Complement Altern Med. 2013. PMID: 23298265 Free PMC article.
-
A molecular insight of inflammatory cascades in rheumatoid arthritis and anti-arthritic potential of phytoconstituents.Mol Biol Rep. 2022 Mar;49(3):2375-2391. doi: 10.1007/s11033-021-06986-7. Epub 2021 Nov 24. Mol Biol Rep. 2022. PMID: 34817776 Review.
-
The Notch signaling-regulated angiogenesis in rheumatoid arthritis: pathogenic mechanisms and therapeutic potentials.Front Immunol. 2023 Oct 26;14:1272133. doi: 10.3389/fimmu.2023.1272133. eCollection 2023. Front Immunol. 2023. PMID: 38022508 Free PMC article. Review.
Cited by
-
A 1, 4-benzoquinone derivative isolated from Ardisia crispa (Thunb.) A. DC. root suppresses angiogenesis via its angiogenic signaling cascades.Saudi Pharm J. 2024 Jan;32(1):101891. doi: 10.1016/j.jsps.2023.101891. Epub 2023 Dec 1. Saudi Pharm J. 2024. PMID: 38111673 Free PMC article.
-
The complete chloroplast genome sequence of Ardisia crispa Thunb.Mitochondrial DNA B Resour. 2022 Dec 9;7(12):2070-2072. doi: 10.1080/23802359.2022.2154621. eCollection 2022. Mitochondrial DNA B Resour. 2022. PMID: 36518737 Free PMC article.
References
-
- Xiang G, Jinyu J, Zhitao F, Xiaoqiang H, Yanan L, Zhigang M. A network pharmacology approach to explore the potential targets underlying the effect of sinomenine on rheumatoid arthritis. Int Immunopharmacol. 2020;80(106201). 10.1016/j.intimp.2020.106201. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

