Spatial architecture of the immune microenvironment orchestrates tumor immunity and therapeutic response
- PMID: 34172088
- PMCID: PMC8234625
- DOI: 10.1186/s13045-021-01103-4
Spatial architecture of the immune microenvironment orchestrates tumor immunity and therapeutic response
Abstract
Tumors are not only aggregates of malignant cells but also well-organized complex ecosystems. The immunological components within tumors, termed the tumor immune microenvironment (TIME), have long been shown to be strongly related to tumor development, recurrence and metastasis. However, conventional studies that underestimate the potential value of the spatial architecture of the TIME are unable to completely elucidate its complexity. As innovative high-flux and high-dimensional technologies emerge, researchers can more feasibly and accurately detect and depict the spatial architecture of the TIME. These findings have improved our understanding of the complexity and role of the TIME in tumor biology. In this review, we first epitomized some representative emerging technologies in the study of the spatial architecture of the TIME and categorized the description methods used to characterize these structures. Then, we determined the functions of the spatial architecture of the TIME in tumor biology and the effects of the gradient of extracellular nonspecific chemicals (ENSCs) on the TIME. We also discussed the potential clinical value of our understanding of the spatial architectures of the TIME, as well as current limitations and future prospects in this novel field. This review will bring spatial architectures of the TIME, an emerging dimension of tumor ecosystem research, to the attention of more researchers and promote its application in tumor research and clinical practice.
Keywords: Immunotherapy; Spatial architecture; Tumor immune microenvironment; Tumor immunity.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
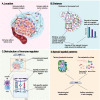


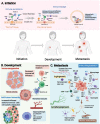

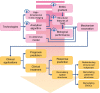
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

