Systemic regulation of mitochondria by germline proteostasis prevents protein aggregation in the soma of C. elegans
- PMID: 34172445
- PMCID: PMC8232903
- DOI: 10.1126/sciadv.abg3012
Systemic regulation of mitochondria by germline proteostasis prevents protein aggregation in the soma of C. elegans
Abstract
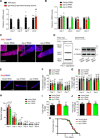
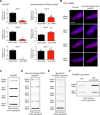
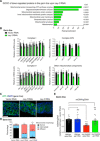
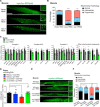
Protein aggregation causes intracellular changes in neurons, which elicit signals to modulate proteostasis in the periphery. Beyond the nervous system, a fundamental question is whether other organs also communicate their proteostasis status to distal tissues. Here, we examine whether proteostasis of the germ line influences somatic tissues. To this end, we induce aggregation of germline-specific PGL-1 protein in germline stem cells of Caenorhabditis elegans Besides altering the intracellular mitochondrial network of germline cells, PGL-1 aggregation also reduces the mitochondrial content of somatic tissues through long-range Wnt signaling pathway. This process induces the unfolded protein response of the mitochondria in the soma, promoting somatic mitochondrial fragmentation and aggregation of proteins linked with neurodegenerative diseases such as Huntington's and amyotrophic lateral sclerosis. Thus, the proteostasis status of germline stem cells coordinates mitochondrial networks and protein aggregation through the organism.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures



Similar articles
-
Germline stem cell arrest inhibits the collapse of somatic proteostasis early in Caenorhabditis elegans adulthood.Aging Cell. 2013 Oct;12(5):814-22. doi: 10.1111/acel.12110. Epub 2013 Jun 28. Aging Cell. 2013. PMID: 23734734
-
Reproductive regulation of the mitochondrial stress response in Caenorhabditis elegans.Cell Rep. 2024 Jun 25;43(6):114336. doi: 10.1016/j.celrep.2024.114336. Epub 2024 Jun 7. Cell Rep. 2024. PMID: 38852157
-
Homeodomain-interacting protein kinase maintains neuronal homeostasis during normal Caenorhabditis elegans aging and systemically regulates longevity from serotonergic and GABAergic neurons.Elife. 2023 Jun 20;12:e85792. doi: 10.7554/eLife.85792. Elife. 2023. PMID: 37338980 Free PMC article.
-
Mediation of organismal aging and somatic proteostasis by the germline.Front Mol Biosci. 2015 Jan 23;2:3. doi: 10.3389/fmolb.2015.00003. eCollection 2015. Front Mol Biosci. 2015. PMID: 25988171 Free PMC article. Review.
-
The heat shock response in neurons and astroglia and its role in neurodegenerative diseases.Mol Neurodegener. 2017 Sep 18;12(1):65. doi: 10.1186/s13024-017-0208-6. Mol Neurodegener. 2017. PMID: 28923065 Free PMC article. Review.
Cited by
-
Behavioral screening of conserved RNA-binding proteins reveals CEY-1/YBX RNA-binding protein dysfunction leads to impairments in memory and cognition.bioRxiv [Preprint]. 2024 May 2:2024.01.05.574402. doi: 10.1101/2024.01.05.574402. bioRxiv. 2024. PMID: 38260399 Free PMC article. Preprint.
-
Regulation of germline proteostasis by HSF1 and insulin/IGF-1 signaling.Biochem Soc Trans. 2023 Apr 26;51(2):501-512. doi: 10.1042/BST20220616. Biochem Soc Trans. 2023. PMID: 36892215 Free PMC article.
-
The killifish germline regulates longevity and somatic repair in a sex-specific manner.Nat Aging. 2024 Jun;4(6):791-813. doi: 10.1038/s43587-024-00632-0. Epub 2024 May 15. Nat Aging. 2024. PMID: 38750187
-
Germline status and micronutrient availability regulate a somatic mitochondrial quality control pathway via short-chain fatty acid metabolism.bioRxiv [Preprint]. 2024 May 21:2024.05.20.594820. doi: 10.1101/2024.05.20.594820. bioRxiv. 2024. PMID: 38826313 Free PMC article. Preprint.
-
A nucleolar mechanism suppresses organismal proteostasis by modulating TGFβ/ERK signalling.Nat Cell Biol. 2025 Jan;27(1):87-102. doi: 10.1038/s41556-024-01564-y. Epub 2025 Jan 3. Nat Cell Biol. 2025. PMID: 39753948
References
-
- Hipp M. S., Kasturi P., Hartl F. U., The proteostasis network and its decline in ageing. Nat. Rev. Mol. Cell Biol. 20, 421–435 (2019). - PubMed
-
- Vilchez D., Saez I., Dillin A., The role of protein clearance mechanisms in organismal ageing and age-related diseases. Nat. Commun. 5, 5659 (2014). - PubMed
-
- Brandt C., Nolte H., Henschke S., Engstrom Ruud L., Awazawa M., Morgan D. A., Gabel P., Sprenger H. G., Hess M. E., Gunther S., Langer T., Rahmouni K., Fenselau H., Krüger M., Brüning J. C., Food perception primes hepatic ER homeostasis via melanocortin-dependent control of mTOR activation. Cell 175, 1321–1335.e20 (2018). - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

