Structure of the mycobacterial ESX-5 type VII secretion system pore complex
- PMID: 34172453
- PMCID: PMC8232910
- DOI: 10.1126/sciadv.abg9923
Structure of the mycobacterial ESX-5 type VII secretion system pore complex
Abstract
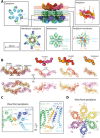
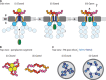
The ESX-5 type VII secretion system is a membrane-spanning protein complex key to the virulence of mycobacterial pathogens. However, the overall architecture of the fully assembled translocation machinery and the composition of the central secretion pore have remained unknown. Here, we present the high-resolution structure of the 2.1-megadalton ESX-5 core complex. Our structure captured a dynamic, secretion-competent conformation of the pore within a well-defined transmembrane section, sandwiched between two flexible protein layers at the cytosolic entrance and the periplasmic exit. We propose that this flexibility endows the ESX-5 machinery with large conformational plasticity required to accommodate targeted protein secretion. Compared to known secretion systems, a highly dynamic state of the pore may represent a fundamental principle of bacterial secretion machineries.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution License 4.0 (CC BY).
Figures


References
-
- I. Kecerdasan, P. Ikep, Global Tuberculosis Report (World Health Organization, 2020).
-
- Orgeur M., Brosch R., Evolution of virulence in the Mycobacterium tuberculosis complex. Curr. Opin. Microbiol. 41, 68–75 (2018). - PubMed
-
- Gey van Pittius N. C., Sampson S. L., Lee H., Kim Y., van Helden P. D., Warren R. M., Evolution and expansion of the Mycobacterium tuberculosis PE and PPE multigene families and their association with the duplication of the ESAT-6 (esx) gene cluster regions. BMC Evol. Biol. 6, 95 (2006). - PMC - PubMed
-
- Ates L. S., van der Woude A. D., Bestebroer J., van Stempvoort G., Musters R. J. P., Garcia-Vallejo J. J., Picavet D. I., van de Weerd R., Maletta M., Kuijl C. P., van der Wel N. N., Bitter W., The ESX-5 system of pathogenic mycobacteria is involved in capsule integrity and virulence through its substrate PPE10. PLOS Pathog. 12, e1005696 (2016). - PMC - PubMed
-
- Ates L. S., Ummels R., Commandeur S., van der Weerd R., Sparrius M., Weerdenburg E., Alber M., Kalscheuer R., Piersma S. R., Abdallah A. M., Abd El Ghany M., Abdel-Haleem A. M., Pain A., Jiménez C. R., Bitter W., Houben E. N. G., Essential role of the ESX-5 secretion system in outer membrane permeability of pathogenic Mycobacteria. PLOS Genet. 11, e1005190 (2015). - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

