Blood eosinophil counts in the general population and airways disease: a comprehensive review and meta-analysis
- PMID: 34172466
- PMCID: PMC8756293
- DOI: 10.1183/13993003.04590-2020
Blood eosinophil counts in the general population and airways disease: a comprehensive review and meta-analysis
Abstract
Background: The clinical context for using blood eosinophil (EOS) counts as treatment-response biomarkers in asthma and COPD requires better understanding of EOS distributions and ranges. We describe EOS distributions and ranges published in asthma, COPD, control (non-asthma/COPD) and general populations.
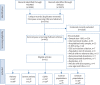
Methods: We conducted a comprehensive literature review and meta-analysis of observational studies (January 2008 to November 2018) that included EOS counts in asthma, severe asthma, COPD, control and general populations. Excluded studies had total sample sizes <200, EOS as inclusion criterion, hospitalised population only and exclusively paediatric participants.
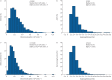
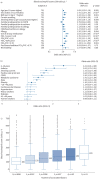
Results: Overall, 91 eligible studies were identified, most had total-population-level data available: asthma (39 studies), severe asthma (12 studies), COPD (23 studies), control (seven studies) and general populations (14 studies); some articles reported data for multiple populations. Reported EOS distributions were right-skewed (seven studies). Reported median EOS counts ranged from 157-280 cells·µL-1 (asthma, 22 studies); 200-400 cells·µL-1 (severe asthma, eight studies); 150-183 cells·µL-1 (COPD, six studies); and 100-160 cells·µL-1 (controls, three studies); and 100-200 cells·µL-1 (general populations, six studies). The meta-analysis showed that observed variability was mostly between studies rather than within studies. Factors reportedly associated with higher blood EOS counts included current smoking, positive skin-prick test, elevated total IgE, comorbid allergic rhinitis, age ≤18 years, male sex, spirometric asthma/COPD diagnosis, metabolic syndrome and adiposity.
Conclusion: EOS distribution and range varied by study population, and were affected by clinical factors including age, smoking history and comorbidities, which, regardless of severity, should be considered during treatment decision-making.
Copyright ©The authors 2022.
Conflict of interest statement
Conflict of interest: V.S. Benson reports employment by GlaxoSmithKline, and stock/share ownership in GlaxoSmithKline. Conflict of interest: S. Hartl reports unrestricted grants from AstraZeneca, GlaxoSmithKline, Böhringer Ingelheim, Menarini, Chiesi Farma, Pfizer, MSD, Air Liquide, Vivisol for the Ludwig Boltzmann Research Institute of Lung Health supporting the LEAD study. Conflict of interest: N. Barnes reports employment by GlaxoSmithKline, and stock/share ownership in GlaxoSmithKline. Conflict of interest: N. Galwey reports employment by GlaxoSmithKline, and stock/share ownership in GlaxoSmithKline. Conflict of interest: M.K. Van Dyke reports employment by GlaxoSmithKline, and stock/share ownership in GlaxoSmithKline. Conflict of interest: N. Kwon reports employment by GlaxoSmithKline, and stock/share ownership in GlaxoSmithKline.
Figures


Comment in
-
Interpreting blood eosinophil counts in health and obstructive lung disease.Eur Respir J. 2022 Jan 13;59(1):2102180. doi: 10.1183/13993003.02180-2021. Print 2022 Jan. Eur Respir J. 2022. PMID: 35027374 No abstract available.
References
-
- Global Initiative for Chronic Obstructive Lung Disease (GOLD) . Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2021 Report. https://goldcopd.org/wp-content/uploads/2020/11/GOLD-REPORT-2021-v1.1-25... - PubMed
-
- Global Initiative for Asthma (GINA) . Global Strategy for Asthma Management and Prevention. 2020 Report. https://ginasthma.org/wp-content/uploads/2020/04/GINA-2020-full-report_-...
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
