Stopping versus continuing long-term mepolizumab treatment in severe eosinophilic asthma (COMET study)
- PMID: 34172470
- PMCID: PMC8733344
- DOI: 10.1183/13993003.00396-2021
Stopping versus continuing long-term mepolizumab treatment in severe eosinophilic asthma (COMET study)
Abstract
Background: The long-term efficacy and safety of mepolizumab for treatment of severe eosinophilic asthma are well established. Here, we examine the clinical impact of stopping mepolizumab after long-term use.
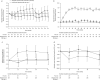
Methods: COMET (NCT02555371) was a randomised, double-blind, placebo-controlled, parallel-group, multicentre study. Patients who had completed COLUMBA (NCT01691859) or COSMEX (NCT02135692) and received continuous mepolizumab treatment for ≥3 years were randomised 1:1 to stop (switch to placebo) or continue subcutaneous mepolizumab 100 mg every 4 weeks for 52 weeks. Primary end-point: time to first clinically significant exacerbation; secondary end-points: time to first exacerbation requiring hospitalisation/emergency department visit, time to decrease in asthma control (≥0.5-point increase in Asthma Control Questionnaire-5 score from COMET baseline) and blood eosinophil count ratio to COMET baseline. Safety was assessed.
Results: Patients stopping (n=151) versus continuing (n=144) mepolizumab had significantly shorter times to first clinically significant exacerbation (hazard ratio 1.61, 95% CI 1.17-2.22; p=0.004) and decrease in asthma control (hazard ratio 1.52, 95% CI 1.13-2.02; p=0.005), and higher blood eosinophil counts at week 52 (270 versus 40 cells·µL-1; ratio (stopping versus continuing) 6.19, 95% CI 4.89-7.83; p<0.001). Differences in efficacy outcomes between groups were observed when assessed from week 12 (16 weeks after last mepolizumab dose). Exacerbations requiring hospitalisation/emergency department visit were rare. Adverse events in patients continuing mepolizumab were consistent with previous studies. For patients who stopped mepolizumab, the safety profile was consistent with other eosinophilic asthma populations.
Conclusion: Patients who stopped mepolizumab had an increase in exacerbations and reduced asthma control versus those who continued.
Copyright ©The authors 2022.
Conflict of interest statement
Conflict of interest: W.C. Moore reports that the study and writing support was funded by GlaxoSmithKline; and has received funding for clinical research and personal fees for participation in advisory boards from GSK, AstraZeneca and Sanofi Regeneron, outside the submitted work. Conflict of interest: O. Kornmann reports that the study and writing support was funded by GlaxoSmithKline; and has received personal fees from AstraZeneca, GSK, Novartis, Boehringer Ingelheim, Sanofi Aventis, and Roche, outside the submitted work. Conflict of interest: M. Humbert reports that the study and writing support was funded by GlaxoSmithKline; and has received personal fees for consultancy services and speaking at conferences, and participation in clinical research projects with AstraZeneca, GSK, Novartis, Roche, Sanofi Regeneron and TEVA; and has a research grant from GSK, outside the submitted work. Conflict of interest: C. Poirier reports that the study and writing support was funded by GlaxoSmithKline; and has received personal fees from GSK, Novartis, Sanofi, and Boehringer Ingelheim, outside the submitted work. Conflict of interest: E.H. Bel reports that the study and writing support was funded by GlaxoSmithKline; and grants from GSK and Teva, personal fees from AstraZeneca, GSK, Novartis, Sanofi/Regeneron, Sterna Biologicals and Chiesi, outside the submitted work. Conflict of interest: N. Kaneko reports that the study and writing support was funded by GlaxoSmithKline. Conflict of interest: S.G. Smith reports that the study and writing support was funded by GlaxoSmithKline; and is an employee of GSK and owns stocks/shares. Conflict of interest: N. Martin reports that the study and writing support was funded by GlaxoSmithKline; and is an employee of GSK and owns stocks/shares. Conflict of interest: M.J. Gilson reports that the study and writing support was funded by GlaxoSmithKline; and is an employee of GSK and owns stocks/shares. Conflict of interest: R.G. Price reports that the study and writing support was funded by GlaxoSmithKline; and is an employee of GSK and owns stocks/shares. Conflict of interest: E.S. Bradford reports that the study and writing support was funded by GlaxoSmithKline; and was an employee at GSK at the time of the study. Conflict of interest: M.C. Liu reports that the study and writing support was funded by GlaxoSmithKline; and has received grants for clinical trials from Boehringer Ingelheim, GSK, MedImmune, Mereo BioPharm, and Gossamer Bio, outside the submitted work.
Figures


References
-
- Chupp GL, Bradford ES, Albers FC, et al. . Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. Lancet Respir Med 2017; 5: 390–400. doi:10.1016/S2213-2600(17)30125-X - DOI - PubMed
