Generation of a safe and efficacious llama single-domain antibody fragment (vHH) targeting the membrane-proximal region of 4-1BB for engineering therapeutic bispecific antibodies for cancer
- PMID: 34172514
- PMCID: PMC8237747
- DOI: 10.1136/jitc-2020-002131
Generation of a safe and efficacious llama single-domain antibody fragment (vHH) targeting the membrane-proximal region of 4-1BB for engineering therapeutic bispecific antibodies for cancer
Abstract
Background: The discovery of checkpoint inhibitors towards cytotoxic T-lymphocyte protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1) has been revolutionary for the treatment of cancers. These therapies have only offered an average of 20%-30% response rates across the tumor spectrum and the combination of agonists towards the tumor-necrosis superfamily members, such as 4-1BB and CD40, has shown potent efficacy in preclinical studies; however, these agonists have exhibited high degrees of toxicity with limited efficacy in human trials. In this study, we have generated a single-domain antibody towards a unique epitope of 4-1BB that limits its potential on-target toxicity while maintaining sufficient potency. This 4-1BB binder is ideal for use in the engineering of multispecific antibodies to localize 4-1BB activation within the tumor microenvironment, as shown here by a anti-PD-L1/4-1BB bispecific candidate (PM1003).
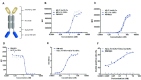
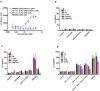
Methods: To determine the functional activity of the 4-1BB- and PD-L1-binding elements of PM1003, in vitro luciferase reporter and primary cell assays were used to test the potency of programmed cell death 1 ligand 1 (PD-L1) blockade and PD-L1-mediated 4-1BB activation via cross-bridging. X-ray crystallography was conducted to resolve the binding epitopes of the respective binding arms, and accurate binding kinetics were determined using standard affinity measurement techniques. Human 4-1BB and/or PD-L1 knock-in mice were used in cancer models for testing the in vivo antitumor efficacy of PM1003, and safety was evaluated further.
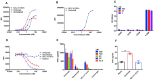
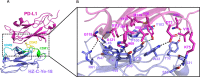
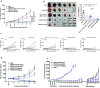
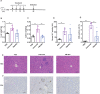
Results: PM1003 shows potent activation of 4-1BB and blockade of PD-L1 in cell-based assays. 4-1BB activation was exerted through the bridging of PD-L1 on target cells and 4-1BB on effector cells. No PD-L1-independent activation of 4-1BB was observed. Through X-ray crystallography, a unique binding epitope in the cysteine-rich domain 4 (CRD4) region was resolved that provides high potency and potentially low on-target toxicity as determined by primary immune cell assays and toxicity evaluation in vivo.
Conclusions: A unique single-domain antibody was discovered that binds to the CRD4 domain of 4-1BB. When incorporated into a 4-1BB/PD-L1 bispecific (PM1003), we have shown the potent inhibition of PD-L1 activity with 4-1BB agonism upon cross-bridging with PD-L1 in vitro. Antitumor activity with minimal toxicity was found in vivo. Thus, PM1003 is a uniquely differentiating and next generation therapeutic agent for cancer therapy.
Keywords: antibody specificity; antigens; differentiation; tumor microenvironment.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: The research was funded by Biotheus Inc. All authors are current employees of Biotheus. Inc, with the exception of JZ, TZ and BL who declare no competing interests.
Figures

Similar articles
-
The enhanced antitumor activity of bispecific antibody targeting PD-1/PD-L1 signaling.Cell Commun Signal. 2024 Mar 12;22(1):179. doi: 10.1186/s12964-024-01562-5. Cell Commun Signal. 2024. PMID: 38475778 Free PMC article. Review.
-
An Fc-muted bispecific antibody targeting PD-L1 and 4-1BB induces antitumor immune activity in colorectal cancer without systemic toxicity.Cell Mol Biol Lett. 2023 May 31;28(1):47. doi: 10.1186/s11658-023-00461-w. Cell Mol Biol Lett. 2023. PMID: 37259060 Free PMC article.
-
Acasunlimab, an Fc-inert PD-L1×4-1BB bispecific antibody, combined with PD-1 blockade potentiates antitumor immunity via complementary immune modulatory effects.J Immunother Cancer. 2025 Apr 10;13(4):e011377. doi: 10.1136/jitc-2024-011377. J Immunother Cancer. 2025. PMID: 40216443 Free PMC article.
-
Novel anti-4-1BB×PD-L1 bispecific antibody augments anti-tumor immunity through tumor-directed T-cell activation and checkpoint blockade.J Immunother Cancer. 2021 Jul;9(7):e002428. doi: 10.1136/jitc-2021-002428. J Immunother Cancer. 2021. PMID: 34230109 Free PMC article.
-
The Next Immune-Checkpoint Inhibitors: PD-1/PD-L1 Blockade in Melanoma.Clin Ther. 2015 Apr 1;37(4):764-82. doi: 10.1016/j.clinthera.2015.02.018. Epub 2015 Mar 29. Clin Ther. 2015. PMID: 25823918 Free PMC article. Review.
Cited by
-
The emerging landscape of novel 4-1BB (CD137) agonistic drugs for cancer immunotherapy.MAbs. 2023 Jan-Dec;15(1):2167189. doi: 10.1080/19420862.2023.2167189. MAbs. 2023. PMID: 36727218 Free PMC article. Review.
-
Combination strategies with PD-1/PD-L1 blockade: current advances and future directions.Mol Cancer. 2022 Jan 21;21(1):28. doi: 10.1186/s12943-021-01489-2. Mol Cancer. 2022. PMID: 35062949 Free PMC article. Review.
-
Small Antibodies with Big Applications: Nanobody-Based Cancer Diagnostics and Therapeutics.Cancers (Basel). 2023 Nov 29;15(23):5639. doi: 10.3390/cancers15235639. Cancers (Basel). 2023. PMID: 38067344 Free PMC article. Review.
-
A bispecific antibody AP203 targeting PD-L1 and CD137 exerts potent antitumor activity without toxicity.J Transl Med. 2023 May 25;21(1):346. doi: 10.1186/s12967-023-04193-5. J Transl Med. 2023. PMID: 37226226 Free PMC article.
-
The enhanced antitumor activity of bispecific antibody targeting PD-1/PD-L1 signaling.Cell Commun Signal. 2024 Mar 12;22(1):179. doi: 10.1186/s12964-024-01562-5. Cell Commun Signal. 2024. PMID: 38475778 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
