Ion-dependent protein-surface interactions from intrinsic solvent response
- PMID: 34172582
- PMCID: PMC8255788
- DOI: 10.1073/pnas.2025121118
Ion-dependent protein-surface interactions from intrinsic solvent response
Abstract
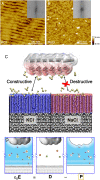
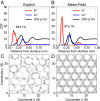
The phyllosilicate mineral muscovite mica is widely used as a surface template for the patterning of macromolecules, yet a molecular understanding of its surface chemistry under varying solution conditions, required to predict and control the self-assembly of adsorbed species, is lacking. We utilize all-atom molecular dynamics simulations in conjunction with an electrostatic analysis based in local molecular field theory that affords a clean separation of long-range and short-range electrostatics. Using water polarization response as a measure of the electric fields that arise from patterned, surface-bound ions that direct the adsorption of charged macromolecules, we apply a Landau theory of forces induced by asymmetrically polarized surfaces to compute protein-surface interactions for two muscovite-binding proteins (DHR10-mica6 and C98RhuA). Comparison of the pressure between surface and protein in high-concentration KCl and NaCl aqueous solutions reveals ion-specific differences in far-field protein-surface interactions, neatly capturing the ability of ions to modulate the surface charge of muscovite that in turn selectively attracts one binding face of each protein over all others.
Keywords: Landau theory; electrostatics; soft matter; solution assembly; specific ion effects.
Conflict of interest statement
The authors declare no competing interest.
Figures

 ,
,  ), (
), ( ,
,  ), (
), ( ,
,  ), (
), ( ,
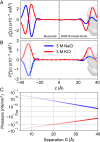
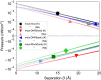
,  ), listed in KCl/NaCl order. For example, the red pair of traces (
), listed in KCl/NaCl order. For example, the red pair of traces ( ,
,  ) corresponds to those in Fig. 4C. With indicating repulsion we see muscovite–muscovite is repulsive under both KCl and NaCl, muscovite–Rhu is attractive in both cases, and the muscovite–DHR interaction can be modulated to attractive or repulsive depending on which protein face is presented and the salt treatment. See
) corresponds to those in Fig. 4C. With indicating repulsion we see muscovite–muscovite is repulsive under both KCl and NaCl, muscovite–Rhu is attractive in both cases, and the muscovite–DHR interaction can be modulated to attractive or repulsive depending on which protein face is presented and the salt treatment. See References
-
- Sievers C., et al. , Phenomena affecting catalytic reactions at solid-liquid interfaces. ACS Catal. 6, 8286–8307 (2016).
-
- Nudelman F., Sommerdijk N. A., Biomineralization as an inspiration for materials chemistry. Angew. Chem. Int. Ed. 51, 6582–6596 (2012). - PubMed

