TNF-α induces endothelial-mesenchymal transition promoting stromal development of pancreatic adenocarcinoma
- PMID: 34172716
- PMCID: PMC8233393
- DOI: 10.1038/s41419-021-03920-4
TNF-α induces endothelial-mesenchymal transition promoting stromal development of pancreatic adenocarcinoma
Abstract
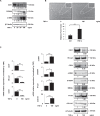
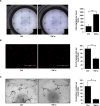
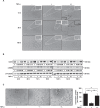
Endothelial-mesenchymal transition (EndMT) is an important source of cancer-associated fibroblasts (CAFs), which facilitates tumour progression. PDAC is characterised by abundant CAFs and tumour necrosis factor-α (TNF-α). Here, we show that TNF-α strongly induces human endothelial cells to undergo EndMT. Interestingly, TNF-α strongly downregulates the expression of the endothelial receptor TIE1, and reciprocally TIE1 overexpression partially prevents TNF-α-induced EndMT, suggesting that TNF-α acts, at least partially, through TIE1 regulation in this process. We also show that TNF-α-induced EndMT is reversible. Furthermore, TNF-α treatment of orthotopic mice resulted in an important increase in the stroma, including CAFs. Finally, secretome analysis identified TNFSF12, as a regulator that is also present in PDAC patients. With the aim of restoring normal angiogenesis and better access to drugs, our results support the development of therapies targeting CAFs or inducing the EndMT reversion process in PDAC.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

