Immunological considerations and challenges for regenerative cellular therapies
- PMID: 34172826
- PMCID: PMC8233383
- DOI: 10.1038/s42003-021-02237-4
Immunological considerations and challenges for regenerative cellular therapies
Abstract
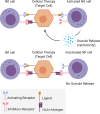
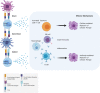
The central goal of regenerative medicine is to replace damaged or diseased tissue with cells that integrate and function optimally. The capacity of pluripotent stem cells to produce unlimited numbers of differentiated cells is of considerable therapeutic interest, with several clinical trials underway. However, the host immune response represents an important barrier to clinical translation. Here we describe the role of the host innate and adaptive immune responses as triggers of allogeneic graft rejection. We discuss how the immune response is determined by the cellular therapy. Additionally, we describe the range of available in vitro and in vivo experimental approaches to examine the immunogenicity of cellular therapies, and finally we review potential strategies to ameliorate immune rejection. In conclusion, we advocate establishment of platforms that bring together the multidisciplinary expertise and infrastructure necessary to comprehensively investigate the immunogenicity of cellular therapies to ensure their clinical safety and efficacy.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
A strategy to protect off-the-shelf cell therapy products using virus-specific T-cells engineered to eliminate alloreactive T-cells.J Transl Med. 2019 Jul 24;17(1):240. doi: 10.1186/s12967-019-1988-y. J Transl Med. 2019. PMID: 31340822 Free PMC article.
-
Immunogenicity of pluripotent stem cells and their derivatives.Circ Res. 2013 Feb 1;112(3):549-61. doi: 10.1161/CIRCRESAHA.111.249243. Circ Res. 2013. PMID: 23371903 Free PMC article. Review.
-
Humanized Mouse Model as a Novel Approach in the Assessment of Human Allogeneic Responses in Organ Transplantation.Front Immunol. 2021 Jun 11;12:687715. doi: 10.3389/fimmu.2021.687715. eCollection 2021. Front Immunol. 2021. PMID: 34177940 Free PMC article.
-
Emerging immunomodulatory strategies for cell therapeutics.Trends Biotechnol. 2023 Mar;41(3):358-373. doi: 10.1016/j.tibtech.2022.11.008. Epub 2022 Dec 20. Trends Biotechnol. 2023. PMID: 36549959 Review.
-
Avoiding immunological rejection in regenerative medicine.Regen Med. 2015;10(3):287-304. doi: 10.2217/rme.15.11. Regen Med. 2015. PMID: 25933238 Review.
Cited by
-
Anti-aging Metabolite-Based Polymeric Microparticles for Intracellular Drug Delivery and Bone Regeneration.Small Sci. 2024 Oct 6;4(10):2400201. doi: 10.1002/smsc.202400201. Epub 2024 Sep 3. Small Sci. 2024. PMID: 39386061 Free PMC article.
-
Stem cell therapy: a revolutionary cure or a pandora's box.Stem Cell Res Ther. 2025 May 22;16(1):255. doi: 10.1186/s13287-025-04334-1. Stem Cell Res Ther. 2025. PMID: 40405306 Free PMC article. Review.
-
Bioengineering solutions for ureteric disorders: clinical need, challenges and opportunities.BJU Int. 2022 Oct;130(4):408-419. doi: 10.1111/bju.15741. Epub 2022 May 15. BJU Int. 2022. PMID: 35388587 Free PMC article. Review.
-
Genetic Engineering of Immune Evasive Stem Cell-Derived Islets.Transpl Int. 2022 Dec 5;35:10817. doi: 10.3389/ti.2022.10817. eCollection 2022. Transpl Int. 2022. PMID: 36545154 Free PMC article. Review.
-
Basic and Translational Research in Cardiac Repair and Regeneration: JACC State-of-the-Art Review.J Am Coll Cardiol. 2021 Nov 23;78(21):2092-2105. doi: 10.1016/j.jacc.2021.09.019. J Am Coll Cardiol. 2021. PMID: 34794691 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

