Preclinical evaluation of eltrombopag in a PDX model of myelodysplastic syndromes
- PMID: 34172896
- PMCID: PMC8727300
- DOI: 10.1038/s41375-021-01327-w
Preclinical evaluation of eltrombopag in a PDX model of myelodysplastic syndromes
Abstract
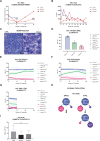
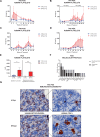
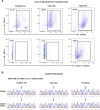
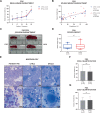
Preclinical research of myelodysplastic syndromes (MDSs) is hampered by a lack of feasible disease models. Previously, we have established a robust patient-derived xenograft (PDX) model for MDS. Here we demonstrate for the first time that this model is applicable as a preclinical platform to address pending clinical questions by interrogating the efficacy and safety of the thrombopoietin receptor agonist eltrombopag. Our preclinical study included n = 49 xenografts generated from n = 9 MDS patient samples. Substance efficacy was evidenced by FACS-based human platelet quantification and clonal bone marrow evolution was reconstructed by serial whole-exome sequencing of the PDX samples. In contrast to clinical trials in humans, this experimental setup allowed vehicle- and replicate-controlled analyses on a patient-individual level deciphering substance-specific effects from natural disease progression. We found that eltrombopag effectively stimulated thrombopoiesis in MDS PDX without adversely affecting the patients' clonal composition. In conclusion, our MDS PDX model is a useful tool for testing new therapeutic concepts in MDS preceding clinical trials.
© 2021. The Author(s).
Conflict of interest statement
D.N. received research funding from Novartis Pharma GmbH. All other authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

